Page 381 - Basic Principles of Textile Coloration
P. 381
370 VAT DYES
deeper shades, but with diminishing returns. The dyeing temperature may be
increased to promote migration, if the dye is stable. This gives better penetration
into tightly constructed materials. Subsequently cooling the dyebath promotes
better exhaustion. Any required salt may be added in portions towards the end of
the process for dyes of lower substantivity.
The liquor ratio is usually in the range from 10:1 to 20:1. The actual amounts
of caustic soda and hydros required for vatting, and for addition to the dyebath,
depend on many factors, such as the dyeing temperature and time, and particularly
on the degree of exposure to air during dyeing. The latter is mainly a characteristic
of the particular dyeing machine used. As in dyeing cellulosic fibres with some
direct dyes, the rate of dyeing with leuco vat dyes is higher at higher temperature,
lower liquor ratio and lower dye concentration. It is not much affected by the
relatively high NaOH and hydros concentrations. Many leuco compounds have
high substantivity and level dyeing requires careful control of the dyeing
temperature, the total salt concentration and gradual addition of the concentrated
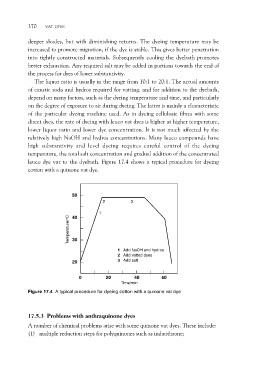
leuco dye vat to the dyebath. Figure 17.4 shows a typical procedure for dyeing
cotton with a quinone vat dye.
50 3
Temperature/oC 2
1
40
30
1 Add NaOH and hydros
2 Add vatted dyes
20 3 Add salt
0 20 40 60
Time/min
Figure 17.4 A typical procedure for dyeing cotton with a quinone vat dye
17.5.3 Problems with anthraquinone dyes
A number of chemical problems arise with some quinone vat dyes. These include:
(1) multiple reduction steps for polyquinones such as indanthrone;