Page 382 - Basic Principles of Textile Coloration
P. 382
DYEING COTTON WITH LEUCO VAT DYES 371
(2) isomerism of leuco compounds to oxanthrones;
(3) hydrolysis of amide groups;
(4) over-oxidation after dyeing;
(5) dehalogenation of some dyes.
To minimise these types of problems, the supplier’s recommendations for vatting
and dyeing must be followed.
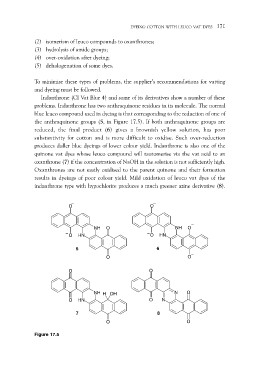
Indanthrone (CI Vat Blue 4) and some of its derivatives show a number of these
problems. Indanthrone has two anthraquinone residues in its molecule. The normal
blue leuco compound used in dyeing is that corresponding to the reduction of one of
the anthraquinone groups (5, in Figure 17.5). If both anthraquinone groups are
reduced, the final product (6) gives a brownish yellow solution, has poor
substantivity for cotton and is more difficult to oxidise. Such over-reduction
produces duller blue dyeings of lower colour yield. Indanthrone is also one of the
quinone vat dyes whose leuco compound will tautomerise via the vat acid to an
oxanthrone (7) if the concentration of NaOH in the solution is not sufficiently high.
Oxanthrones are not easily oxidised to the parent quinone and their formation
results in dyeings of poor colour yield. Mild oxidation of leuco vat dyes of the
indanthrone type with hypochlorite produces a much greener azine derivative (8).
OO
NH O NH O
O HN O HN
5 6
O O
O O
NH H OH NO
ON
O HN
8
7 O
O
Figure 17.5