Page 29 - Electronic Configuration
P. 29
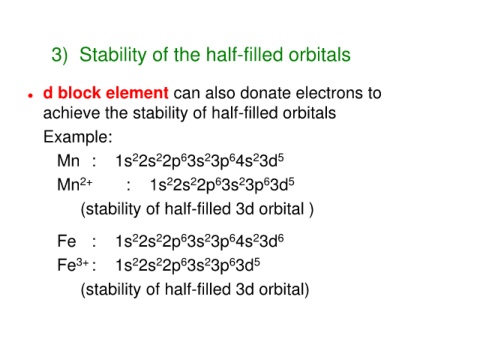
3) Stability of the half-filled orbitals
d block element can also donate electrons to
achieve the stability of half-filled orbitals
Example:
2
6
2
2
2
Mn : 1s 2s 2p 3s 3p 4s 3d 5
6
Mn 2+ : 1s 2s 2p 3s 3p 3d 5
2
2
6
6
2
(stability of half-filled 3d orbital )
6
2
2
2
Fe : 1s 2s 2p 3s 3p 4s 3d 6
6
2
6
2
3+
Fe : 1s 2s 2p 3s 3p 3d 5
6
2
2
(stability of half-filled 3d orbital)