Page 25 - Electronic Configuration
P. 25
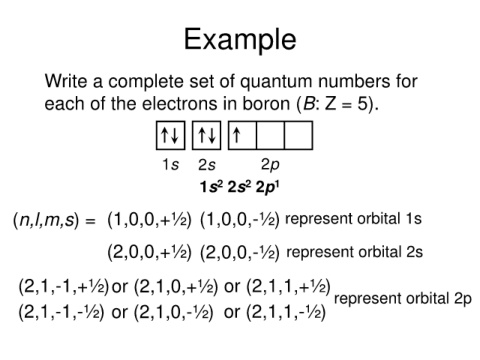
Example
Write a complete set of quantum numbers for
each of the electrons in boron (B: Z = 5).
1s 2s 2p
1s 2s 2p 1
2
2
(n,l,m,s) = (1,0,0,+½) (1,0,0,-½) represent orbital 1s
(2,0,0,+½) (2,0,0,-½) represent orbital 2s
(2,1,-1,+½)or (2,1,0,+½) or (2,1,1,+½)
represent orbital 2p
(2,1,-1,-½) or (2,1,0,-½) or (2,1,1,-½)