Page 26 - Electronic Configuration
P. 26
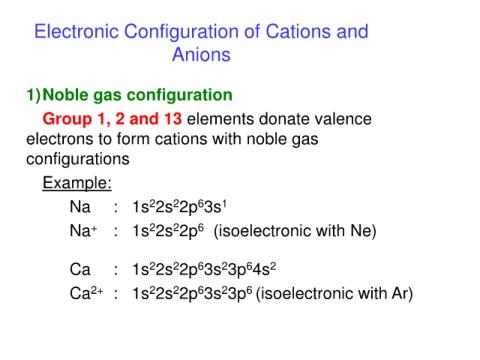
Electronic Configuration of Cations and
Anions
1)Noble gas configuration
Group 1, 2 and 13 elements donate valence
electrons to form cations with noble gas
configurations
Example:
2
2
Na : 1s 2s 2p 3s 1
6
2
6
2
Na + : 1s 2s 2p (isoelectronic with Ne)
Ca : 1s 2s 2p 3s 3p 4s 2
2
6
2
2
6
2
6
2
Ca 2+ : 1s 2s 2p 3s 3p (isoelectronic with Ar)
6
2