Page 563 - fbkCardioDiabetes_2017
P. 563
Cardio Diabetes Medicine 2017 539
BASAL PLUS INSULINS
BASAL PLUS- CLINICAL EVIDENCE
Trial Duration of Trial arms Population HbA1c Target Proportion Severe Hypo- Weight
randomized size change % HbAic achieving glycemia rate change,
treatment {mmol/ target (events / pa- Kg
period mol} HbA1c % tient – year )
OPAL Study(20) 24 weeks Insulin glargine+ 162 -0.36 ≤48 (6.5) 27.8 0.01±0.15 +1.0
OADs+ Insulin
glulisine at break-
fast,
Insulin 154 -0.31 ≤48(6.5) 33.8 0.04±0.30 +0.9
glargine+OADs+-
Insulin glulisine at
main meal time
Proof of concept 3 months Insulin 51 -0.11 ≤53 (<7) 8.8 0.2±1.1 -0.4
study (33) glargine+OADs
Insulin 45 -0.37 ≤53 (<7) 22.4 0.0±0.0 +0.7
glargine+OADs+-
Insulin glulisine at
main meal time
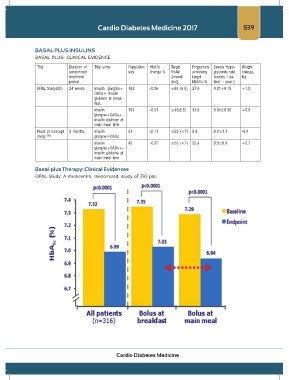
Basal-plus Therapy: Clinical Evidences
OPAL Study: A multicentre, randomized, study of 393 pati
Cardio Diabetes Medicine