Page 245 - Physics Form 5 KSSM_Neat
P. 245
h CHAPTER 7
Formulae such as E = hf and l = , involve Planck's constant, h. How can the value of this
p
constant, h be determined in the laboratory?
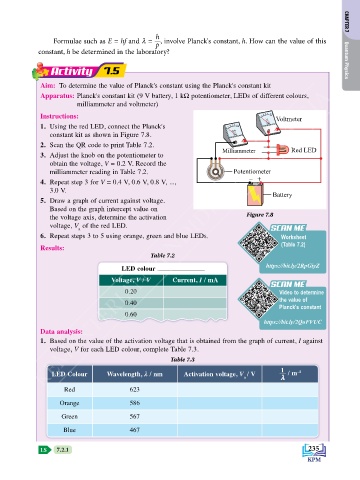
Activity 7.5 Quantum Physics
Aim: To determine the value of Planck's constant using the Planck's constant kit
KEMENTERIAN PENDIDIKAN MALAYSIA
Apparatus: Planck's constant kit (9 V battery, 1 kW potentiometer, LEDs of different colours,
milliammeter and voltmeter)
Instructions: 0 1 2 3 4 5 6
V Voltmeter
1. Using the red LED, connect the Planck's 0 1 2 3 4 5 6
mA
constant kit as shown in Figure 7.8.
2. Scan the QR code to print Table 7.2.
Milliammeter Red LED
3. Adjust the knob on the potentiometer to
obtain the voltage, V = 0.2 V. Record the
milliammeter reading in Table 7.2. Potentiometer
– +
4. Repeat step 3 for V = 0.4 V, 0.6 V, 0.8 V, ...,
3.0 V.
Battery
5. Draw a graph of current against voltage.
Based on the graph intercept value on
the voltage axis, determine the activation Figure 7.8
voltage, V of the red LED.
SCAN ME
a SCAN ME
6. Repeat steps 3 to 5 using orange, green and blue LEDs. Worksheet
(Table 7.2)
Results:
Table 7.2
https://bit.ly/2RpGiyZ
LED colour
Voltage, V / V Current, I / mA
SCAN ME
SCAN ME
0.20 Video to determine
the value of
0.40
Planck's constant
0.60
https://bit.ly/2QoFVUC
Data analysis:
1. Based on the value of the activation voltage that is obtained from the graph of current, I against
voltage, V for each LED colour, complete Table 7.3.
Table 7.3
1 –1
LED Colour Wavelength, λ / nm Activation voltage, V / V / m
a l
Red 623
Orange 586
Green 567
Blue 467
LS 7.2.1 235