Page 67 - Physics Form 5 KSSM_Neat
P. 67
Activity 2.5 CHAPTER 2
Aim: To determine the pressure of a gas using a water manometer Pressure
Apparatus: Manometer, rubber tube, half-metre rule, 10 ml plastic syringe
Materials: Water and red colouring
Instructions:
KEMENTERIAN PENDIDIKAN MALAYSIA
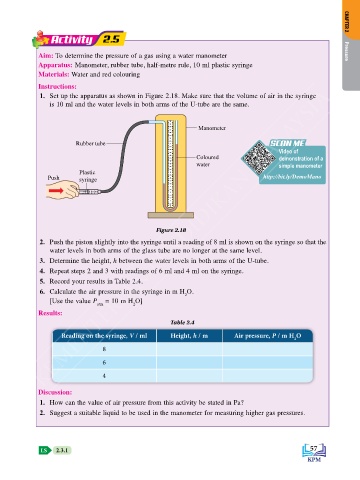
1. Set up the apparatus as shown in Figure 2.18. Make sure that the volume of air in the syringe
is 10 ml and the water levels in both arms of the U-tube are the same.
30
29
28 Manometer
27
26
25
24
SCAN ME
Rubber tube 23 22 SCAN ME
21
20 Video of
19
18
17 Coloured demonstration of a
16
15 water simple manometer
14
13
Plastic 12 11
Push syringe 10 9 http://bit.ly/DemoMano
8
7
6
5
4
2
1
3
5
4
3
2
1
cm
0
Figure 2.18
2. Push the piston slightly into the syringe until a reading of 8 ml is shown on the syringe so that the
water levels in both arms of the glass tube are no longer at the same level.
3. Determine the height, h between the water levels in both arms of the U-tube.
4. Repeat steps 2 and 3 with readings of 6 ml and 4 ml on the syringe.
5. Record your results in Table 2.4.
6. Calculate the air pressure in the syringe in m H O.
2
[Use the value P = 10 m H O]
atm 2
Results:
Table 2.4
Reading on the syringe, V / ml Height, h / m Air pressure, P / m H O
2
8
6
4
Discussion:
1. How can the value of air pressure from this activity be stated in Pa?
2. Suggest a suitable liquid to be used in the manometer for measuring higher gas pressures.
LS 2.3.1 57