Page 68 - Physics Form 5 KSSM_Neat
P. 68
Solving Problems in Daily Life Involving Gas Pressure
Example 1
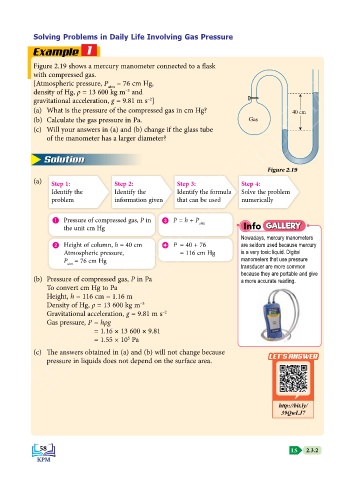
Figure 2.19 shows a mercury manometer connected to a flask
with compressed gas.
[Atmospheric pressure, P = 76 cm Hg,
atm
KEMENTERIAN PENDIDIKAN MALAYSIA
–3
density of Hg, ρ = 13 600 kg m and
–2
gravitational acceleration, g = 9.81 m s ]
(a) What is the pressure of the compressed gas in cm Hg? 40 cm
(b) Calculate the gas pressure in Pa. Gas
(c) Will your answers in (a) and (b) change if the glass tube
of the manometer has a larger diameter?
Solution
Figure 2.19
(a) Step 1: Step 2: Step 3: Step 4:
Identify the Identify the Identify the formula Solve the problem
problem information given that can be used numerically
Pressure of compressed gas, P in 3 P = h + P atm
the unit cm Hg Info GALLERY
Nowadays, mercury manometers
2 Height of column, h = 40 cm P = 40 + 76 are seldom used because mercury
Atmospheric pressure, = 116 cm Hg is a very toxic liquid. Digital
P = 76 cm Hg manometers that use pressure
atm transducer are more common
because they are portable and give
(b) Pressure of compressed gas, P in Pa a more accurate reading.
To convert cm Hg to Pa
Height, h = 116 cm = 1.16 m
Density of Hg, ρ = 13 600 kg m –3
Gravitational acceleration, g = 9.81 m s –2
Gas pressure, P = hρg
= 1.16 × 13 600 × 9.81
= 1.55 × 10 Pa
5
(c) The answers obtained in (a) and (b) will not change because LET’S ANSWER
LET’S ANSWER
pressure in liquids does not depend on the surface area.
http://bit.ly/
39QwLJ7
58 LS 2.3.2