Page 968 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 968
CHAPTER 75: Urinary Tract Infections 699
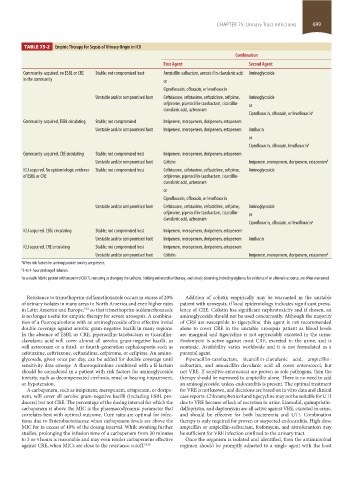
TABLE 75-2 Empiric Therapy for Sepsis of Urinary Origin in ICU
Combination
First Agent Second Agent
Community-acquired, no ESBL or CRE Stable; not compromised host Ampicillin-sulbactam, amoxicillin-clavulanic acid Aminoglycoside
in the community or
Ciprofloxacin, ofloxacin, or levofloxacin
Unstable and/or compromised host Ceftriaxone, cefotaxime, ceftazidime, cefipime, Aminoglycoside
cefpirome, piperacillin-tazobactam, ticarcillin- or
clavulanic acid, aztreonam
Ciprofloxacin, ofloxacin, or levofloxacin a
Community-acquired, ESBL circulating Stable; not compromised Imipenem, meropenem, doripenem, ertapenem
Unstable and/or compromised host Imipenem, meropenem, doripenem, ertapenem Amikacin
or
Ciprofloxacin, ofloxacin, levofloxacin b
Community-acquired, CRE circulating Stable; not compromised host Imipenem, meropenem, doripenem, ertapenem
Unstable and/or compromised host Colistin Imipenem, meropenem, doripenem, ertapenem b
ICU acquired. No epidemiologic evidence Stable; not compromised host Ceftriaxone, cefotaxime, ceftazidime, cefipime, Aminoglycoside
of ESBL or CRE cefpirome, piperacillin-tazobactam, ticarcillin-
clavulanic acid, aztreonam
or
Ciprofloxacin, ofloxacin, or levofloxacin
Unstable and/or compromised host Ceftriaxone, cefotaxime, ceftazidime, cefipime, Aminoglycoside
cefpirome, piperacillin-tazobactam, ticarcillin- or
clavulanic acid, aztreonam
Ciprofloxacin, ofloxacin, or levofloxacin a
ICU acquired. ESBL circulating Stable; not compromised host Imipenem, meropenem, doripenem, ertapenem c
Unstable and/or compromised host Imipenem, meropenem, doripenem, ertapenem Amikacin
ICU acquired. CRE circulating Stable; not compromised host Imipenem, meropenem, doripenem, ertapenem
Unstable and/or compromised host Colistin Imipenem, meropenem, doripenem, ertapenem b
a When risk factors for aminoglycoside toxicity are present.
b 3-to 4-hour prolonged infusion.
c In a stable febrile patient with presumed CAUTI, removing or changing the catheter, holding antimicrobial therapy, and closely observing, including vigilance for evidence of an alternative source, are often warranted.
Resistance to trimethoprim-sulfamethoxazole occurs in excess of 20% Addition of colistin empirically may be warranted in the unstable
of urinary isolates in many areas in North America and even higher rates patient with urosepsis, if local epidemiology indicates significant preva-
in Latin America and Europe, so that trimethoprim-sulfamethoxazole lence of CRE. Colistin has significant nephrotoxicity and if chosen, an
7,53
is no longer useful for empiric therapy for severe urosepsis. A combina- aminoglycoside should not be used concurrently. Although the majority
tion of a fluoroquinolone with an aminoglycoside offers effective initial of CRE are susceptible to tigecycline, this agent is not recommended
double coverage against aerobic gram-negative bacilli in many regions. alone to cover CRE in the unstable urosepsis patient as blood levels
In the absence of ESBL or CRE, piperacillin-tazobactam or ticarcillin- are marginal and tigecycline is not appreciably excreted in the urine.
clavulanic acid will cover almost all aerobic gram-negative bacilli, as Fosfomycin is active against most CRE, excreted in the urine, and is
will aztreonam or a third- or fourth-generation cephalosporin such as nontoxic. Availability varies worldwide and it is not formulated as a
cefotaxime, ceftriaxone, ceftazidime, cefpirome, or cefipime. An amino- parental agent.
glycoside, given once per day, can be added for double coverage until Piperacillin-tazobactam, ticarcillin-clavulanic acid, ampicillin-
sensitivity data emerge. A fluoroquinolone combined with a ß-lactam sulbactam, and amoxicillin-clavulanic acid all cover enterococci, but
should be considered in a patient with risk factors for aminoglycoside not VRE. If sensitive enterococci are proven as sole pathogens, then the
toxicity, such as decompensated cirrhosis, renal or hearing impairment, therapy should be narrowed to ampicillin alone. There is no need to add
or hypotension. an aminoglycoside, unless endocarditis is present. The optimal treatment
A carbapenem, such as imipenem, meropenem, ertapenem, or doripe- for VRE is not known, and decisions are based on in vitro data and clinical
nem, will cover all aerobic gram-negative bacilli (including ESBL pro- case reports. Chloramphenicol and tigecycline may not be suitable for UTI
ducers) but not CRE. The percentage of the dosing interval for which the due to VRE because of lack of excretion in urine. Linezolid, quinupristin-
carbapenem is above the MIC is the pharmacodynamic parameter that dalfopristin, and daptomycin are all active against VRE, excreted in urine,
correlates best with optimal outcome. Cure rates are optimal for infec- and should be effective for both bacteremia and UTI. Combination
tions due to Enterobacteriaceae when carbapenem levels are above the therapy is only required for proven or suspected endocarditis. High-dose
MIC for in excess of 40% of the dosing interval. While awaiting further ampicillin or ampicillin-sulbactam, fosfomycin, and nitrofurantoin may
studies, prolonging the infusion time of a carbapenem from 30 minutes be sufficient for VRE infection confined to the urinary tract.
to 3 or 4 hours is reasonable and may even render carbapenems effective Once the organism is isolated and identified, then the antimicrobial
against CRE, when MICs are close to the resistance cutoff. 54,55 regimen should be promptly adjusted to a single agent with the least
section05_c74-81.indd 699 1/23/2015 12:37:27 PM