Page 991 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 991
722 PART 5: Infectious Disorders
THE BIOLOGY OF MALARIAL PARASITES When infected mosquitoes bite humans (Fig. 78-2), needle-shaped
sporozoite stages of infection (approximately half a dozen) are injected
Since antiquity malaria has been a potent selective force on humanity’s and migrate within an hour to the liver to begin a clinically silent stage of
history and its genes. Some Egyptian pharaohs carried malarial parasites intense multiplication within hepatocytes. One sporozoite can produce
2
1
and there are many descriptions of fevers in ancient cultures that are tens of thousands of progeny within 5 to 16 days, depending on species;
consistent with the diagnosis of malaria. Alphonse Laveran in Algiers in the median time for P falciparum is 6 days. These progeny are then
1880 discovered that protozoan parasites cause malaria. Since then, four released into the bloodstream and multiply by asexual division in red
3
species of Plasmodium: P falciparum, P vivax, P malariae, and P ovale are cells until after several cycles of logarithmic growth sufficient parasites
established as causes of natural human malaria infections, with a fifth are generated to result in symptoms of disease. The numbers of parasites
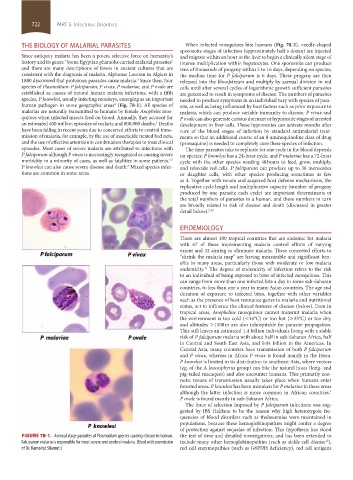
species, P knowlesi, usually infecting monkeys, emerging as an important needed to produce symptoms in an individual vary with species of para-
human pathogen in some geographic areas (Fig. 78-1). All species of site, as well as being influenced by host factors such as prior exposure to
4
malarias are naturally transmitted to humans by female Anopheles mos- malaria, which can produce variable immunity to disease. P vivax and
quitoes when infected insects feed on blood. Annually, they account for P ovale can also generate curious dormant or hypnozoite stages of arrested
an estimated 400 million episodes of malaria and 800,000 deaths. Deaths development in liver cells. These hypnozoites can activate months after
5
have been falling in recent years due to concerted efforts to control trans- cure of the blood stages of infection by standard antimalarial treat-
mission of malaria, for example, by the use of insecticide treated bed nets, ments so that an additional course of an 8-aminoquinoline class of drug
and the use of effective artemisinin combination therapies to treat clinical ( primaquine) is needed to completely cure these species of infection.
episodes. Most cases of severe malaria are attributed to infections with The time parasites take to replicate for one cycle in the blood depends
P falciparum although P vivax is increasingly recognized as causing severe on species: P knowlesi has a 24-hour cycle, and P malariae has a 72-hour
morbidity in a minority of cases, as well as fatalities in some patients. cycle with the other species needing 48 hours to feed, grow, multiply,
6,7
P knowlesi can also cause severe disease and death. Mixed species infec- and reinvade red cells. P falciparum can produce up to 36 merozoites
8
tions are common in some areas. or daughter cells, with other species producing sometimes as few
as 4. Together with innate and acquired host defense mechanisms, the
replicative cycle length and multiplicative capacity (number of progeny
produced by one parasite each cycle) are important determinants of
the total numbers of parasites in a human, and these numbers in turn
are broadly related to risk of disease and death (discussed in greater
detail below). 9,10
EPIDEMIOLOGY
There are almost 100 tropical countries that are endemic for malaria
with 67 of these implementing malaria control efforts of varying
extent and 32 aiming to eliminate malaria. These concerted efforts to
“shrink the malaria map” are having measurable and significant ben-
efits in many areas, particularly those with moderate or low malaria
endemicity. The degree of endemicity of infection refers to the risk
11
to an individual of being exposed to bites of infected mosquitoes. This
can range from more than one infected bite a day in some sub-Saharan
countries, to less than one a year in many Asian countries. The age and
duration of exposure to infected bites, together with other variables
such as the presence of host resistance genes to malaria and nutritional
status, act to influence the clinical features of disease (below). Even in
tropical areas, Anopheline mosquitoes cannot transmit malaria when
the environment is too cold (<16°C) or too hot (>33°C) or too dry,
and altitudes >1500 m are also inhospitable for parasite propagation.
This still leaves an estimated 1.4 billion individuals living with a stable
risk of P falciparum malaria with about half in sub-Saharan Africa, half
in Central and South East Asia, and 0.04 billion in the Americas. In
Central Asia, many countries have transmission of both P falciparum
and P vivax, whereas in Africa P vivax is found mainly in the Horn.
P knowlesi is limited in its distribution to southeast Asia, where vectors
(eg, of the A leucosphyrus group) can bite the natural hosts (long- and
pig-tailed macaques) and also encounter humans. This primarily zoo-
notic means of transmission usually takes place when humans enter
forested areas. P knowlesi has been mistaken for P malariae in these areas
although the latter infection is more common in African countries.
4
P ovale is found mainly in sub-Saharan Africa.
The force of selection imposed by P falciparum infections was sug-
gested by JBS Haldane to be the reason why high heterozygote fre-
quencies of blood disorders such as thalassemias were maintained in
populations, because these hemoglobinopathies might confer a degree
of protection against sequelae of infection. This hypothesis has stood
FIGURE 78-1. Asexual stage parasites of Plasmodium species causing disease in human. the test of time and detailed investigations, and has been extended to
12
Falciparum malaria is responsible for most severe and cerebral malaria. (Used with permission include many other hemoglobinopathies (such as sickle cell disease ),
of Dr. Kamolrat Silamut.) red cell enzymopathies (such as G6PDH deficiency), red cell antigens
section05_c74-81.indd 722 1/23/2015 12:37:35 PM