Page 992 - Hall et al (2015) Principles of Critical Care-McGraw-Hill
P. 992
CHAPTER 78: Severe Malaria 723
Salivary glands
Sporozoites
~10 Mosquito
Sporozoites
Liver
10 5
RBC
Merozoites
10 8–11 (uncomplicated) Mosquito Oocyst
to 10 12–13 (severe) gut
Trophozoite Ookinete
Zygote
Gametocytes
Gametes
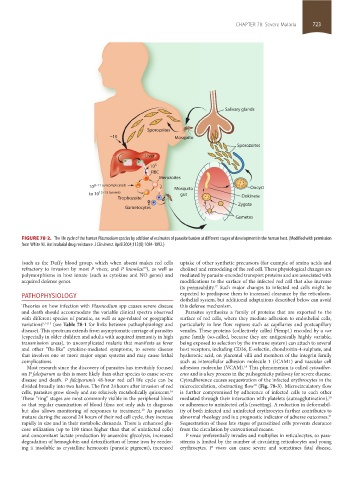
FIGURE 78-2. The life cycle of the human Plasmodium species by addition of estimates of parasite burden at different stages of development in the human host. (Modified with permission
from White NJ. Antimalarial drug resistance. J Clin Invest. April 2004;113(8):1084-1092.)
(such as the Duffy blood group, which when absent makes red cells uptake of other synthetic precursors (for example of amino acids and
refractory to invasion by most P vivax, and P knowlesi ), as well as choline) and remodeling of the red cell. These physiological changes are
13
polymorphisms in host innate (such as cytokine and NO genes) and mediated by parasite-encoded transport proteins and are associated with
acquired defense genes. modifications to the surface of the infected red cell that also increase
its permeability. Such major changes to infected red cells might be
17
PATHOPHYSIOLOGY expected to predispose them to increased clearance by the reticuloen-
dothelial system, but additional adaptations described below can avoid
Theories on how infection with Plasmodium spp causes severe disease this defense mechanism.
and death should accommodate the variable clinical spectra observed Parasites synthesize a family of proteins that are exported to the
with different species of parasite, as well as age-related or geographic surface of red cells, where they mediate adhesion to endothelial cells,
variations 9,14,15 (see Table 78-1 for links between pathophysiology and particularly in low flow regions such as capillaries and postcapillary
disease). This spectrum extends from asymptomatic carriage of parasites venules. These proteins (collectively called Pfemp1) encoded by a var
(especially in older children and adults with acquired immunity in high gene family (so-called, because they are antigenically highly variable,
transmission areas), to uncomplicated malaria that manifests as fever being exposed to selection by the immune system) can attach to several
and other “flu-like” cytokine-mediated symptoms, to severe disease host receptors, including CD36, E-selectin, chondroitin-4-sulphate, and
that involves one or more major organ systems and may cause lethal hyaluronic acid, on placental villi and members of the integrin family
complications. such as intercellular adhesion molecule 1 (ICAM1) and vascular cell
Most research since the discovery of parasites has inevitably focused adhesion molecular (VCAM). This phenomenon is called cytoadher-
18
on P falciparum as this is more likely than other species to cause severe ence and is a key process in the pathogenicity pathway for severe disease.
disease and death. P falciparum’s 48-hour red cell life cycle can be Cytoadherence causes sequestration of the infected erythrocytes in the
divided broadly into two halves. The first 24 hours after invasion of red microcirculation, obstructing flow (Fig. 78-3). Microcirculatory flow
19
cells, parasites grow slowly and are relatively metabolically quiescent. is further compromised by adherence of infected cells to each other
16
These “ring” stages are most commonly visible in the peripheral blood mediated through their interaction with platelets (autoagglutination),
20
so that regular examination of blood films not only aids in diagnosis or adherence to uninfected cells (rosetting). A reduction in deformabil-
but also allows monitoring of responses to treatment. As parasites ity of both infected and uninfected erythrocytes further contributes to
10
mature during the second 24 hours of their red cell cycle, they increase abnormal rheology and is a prognostic indicator of adverse outcomes.
21
rapidly in size and in their metabolic demands. There is enhanced glu- Sequestration of these late stages of parasitized cells prevents clearance
cose utilization (up to 100 times higher than that of uninfected cells) from the circulation by conventional means.
and concomitant lactate production by anaerobic glycolysis, increased P vivax preferentially invades and multiplies in reticulocytes, so para-
degradation of hemoglobin and detoxification of heme iron by render- sitemia is limited by the number of circulating reticulocytes and young
ing it insoluble as crystalline hemozoin (parasite pigment), increased erythrocytes. P vivax can cause severe and sometimes fatal disease,
section05_c74-81.indd 723 1/23/2015 12:37:36 PM