Page 1063 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1063
946 Part VII Hematologic Malignancies
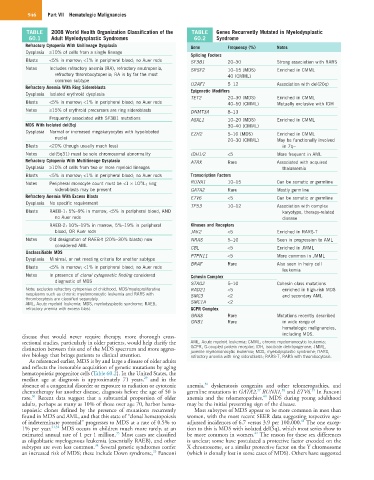
TABLE 2008 World Health Organization Classification of the TABLE Genes Recurrently Mutated in Myelodysplastic
60.1 Adult Myelodysplastic Syndromes 60.2 Syndrome
Refractory Cytopenia With Unilineage Dysplasia Gene Frequency (%) Notes
Dysplasia ≥10% of cells from a single lineage
Splicing Factors
Blasts <5% in marrow; <1% in peripheral blood; no Auer rods SF3B1 20–30 Strong association with RARS
Notes Includes refractory anemia (RA), refractory neutropenia, SRSF2 10–15 (MDS) Enriched in CMML
refractory thrombocytopenia; RA is by far the most 40 (CMML)
common subtype
Refractory Anemia With Ring Sideroblasts U2AF1 5–12 Association with del(20q)
Dysplasia Isolated erythroid dysplasia Epigenetic Modifiers
TET2 20–30 (MDS) Enriched in CMML
Blasts <5% in marrow; <1% in peripheral blood; no Auer rods 40–50 (CMML) Mutually exclusive with IDH
Notes ≥15% of erythroid precursors are ring sideroblasts DNMT3A 8–13
Frequently associated with SF3B1 mutations ASXL1 10–20 (MDS) Enriched in CMML
MDS With Isolated del(5q) 30–40 (CMML)
Dysplasia Normal or increased megakaryocytes with hypolobated EZH2 5–10 (MDS) Enriched in CMML
nuclei
20–30 (CMML) May be functionally involved
Blasts <20% (though usually much less) in 7q−
Notes del(5q31) must be sole chromosomal abnormality IDH1/2 <5 More frequent in AML
Refractory Cytopenia With Multilineage Dysplasia ATRX Rare Associated with acquired
Dysplasia ≥10% of cells from two or more myeloid lineages thalassemia
Blasts <5% in marrow; <1% in peripheral blood; no Auer rods Transcription Factors
9
Notes Peripheral monocyte count must be <1 × 10 /L; ring RUNX1 10–15 Can be somatic or germline
sideroblasts may be present GATA2 Rare Mostly germline
Refractory Anemia With Excess Blasts ETV6 <5 Can be somatic or germline
Dysplasia No specific requirement
TP53 10–12 Association with complex
Blasts RAEB-1: 5%–9% in marrow, <5% in peripheral blood, AND karyotype, therapy-related
no Auer rods disease
RAEB-2: 10%–19% in marrow, 5%–19% in peripheral Kinases and Receptors
blood, OR Auer rods JAK2 <5 Enriched in RARS-T
Notes Old designation of RAEB-t (20%–30% blasts) now NRAS 5–10 Seen in progression to AML
considered AML CBL <5 Enriched in JMML
Unclassifiable MDS
Dysplasia Minimal, or not meeting criteria for another subtype PTPN11 <5 More common in JMML
BRAF Rare Also seen in hairy cell
Blasts <5% in marrow; <1% in peripheral blood; no Auer rods
leukemia
Notes In presence of clonal cytogenetic finding considered Cohesin Complex
diagnostic of MDS
STAG2 5–10 Cohesin class mutations
Note: excludes refractory cytopenias of childhood. MDS/myeloproliferative RAD21 <5 enriched in high-risk MDS
neoplasms such as chronic myelomonocytic leukemia and RARS with SMC3 <2 and secondary AML.
thrombocytosis are classified separately.
AML, Acute myeloid leukemia; MDS, myelodysplastic syndrome; RAEB, SMC1A <2
refractory anemia with excess blast. GCPR Complex
GNAS Rare Mutations recently described
GNB1 Rare in wide range of
hematologic malignancies,
including MDS.
disease that would never require therapy, more thorough cross-
sectional studies, particularly in older patients, would help clarify the AML, Acute myeloid leukemia; CMML, chronic myelomonocytic leukemia;
distinction between this end of the MDS spectrum and more aggres- GCPR, G-coupled protein receptor; IDH, isocitrate dehdryogenase; JMML,
juvenile myelomonocytic leukemia; MDS, myelodysplastic syndrome; RARS,
sive biology that brings patients to clinical attention. refractory anemia with ring sideroblasts; RARS-T, RARS with thrombocytosis.
As referenced earlier, MDS is by and large a disease of older adults
and reflects the inexorable acquisition of genetic mutations by aging
hematopoietic progenitor cells (Table 60.2). In the United States, the
29
median age at diagnosis is approximately 71 years, and in the
36
absence of a congenital disorder or exposure to radiation or cytotoxic anemia, dyskeratosis congenita and other telomeropathies, and
37
38
39
chemotherapy for another disease, diagnosis before the age of 50 is germline mutations in GATA2, RUNX1, and ETV6. In Fanconi
40
30
rare. Recent data suggest that a substantial proportion of older anemia and the telomeropathies, MDS during young adulthood
adults, perhaps as many as 10% of those over age 70, harbor hema- may be the initial presenting sign of the disease.
topoietic clones defined by the presence of mutations recurrently Most subtypes of MDS appear to be more common in men than
found in MDS and AML, and that this state of “clonal hematopoiesis women, with the most recent SEER data suggesting respective age-
41
of indeterminate potential” progresses to MDS at a rate of 0.5% to adjusted incidences of 6.7 versus 3.9 per 100,000. The one excep-
1% per year. 31,32 MDS occurs in children much more rarely, at an tion to this is MDS with isolated del(5q), which most series show to
33
42
estimated annual rate of 1 per 1 million. Most cases are classified be more common in women. The reason for these sex differences
as oligoblastic myelogenous leukemia (essentially RAEB), and other is unclear; some have postulated a protective factor encoded on the
34
subtypes are even less common. Several genetic syndromes confer X chromosome, or a similar protective factor on the Y chromosome
35
an increased risk of MDS; these include Down syndrome, Fanconi (which is clonally lost in some cases of MDS). Others have suggested