Page 1147 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1147
1006 Part VII Hematologic Malignancies
Pediatric acute lymphoblastic leukemia
1.4% LYL1
t(7;19)
2.3% TLX3
0.3% TLX1
7% TAL1
t(1;14), t(1;7), 1 p32del Hyperdiploid 20%
Other >50 chromosomes
4% MYC-Ig Translocations (T cell) 4%
t(8;14), t(2;8), t(8;22) BCR-ABL1
2%
t(9;22)
10% Other (Precursor Bcell)
5% TCF3-PBX1 ETV6-RUNX1
t(1;19) 22%
t(12;21)
1% TCF3-HLF
t(17;19)
MLL fusions 6%
1% Hypodiploid t(1;11), t(4;11), t(11;19)
<44 chromosomes
Ph-Like 10% iAmp21 2%
CRLF2 rearranged 4%
Genetic aberrations in
Ph-Like ALL include:
CRLF2 rearrangement Precursor B cell Mature B cell T cell
ABL1 fusion
JAK2 rearrangement
EPOR rearrangement
RAS mutations
JAK-STAT mutations
A
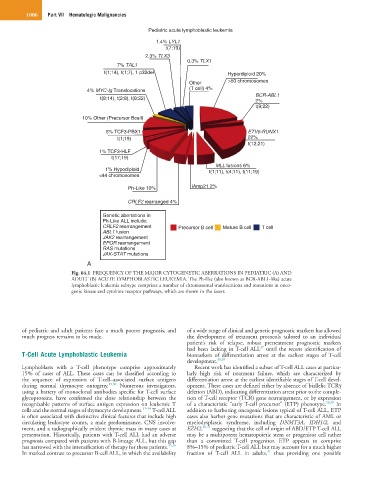
Fig. 64.1 FREQUENCY OF THE MAJOR CYTOGENETIC ABERRATIONS IN PEDIATRIC (A) AND
ADULT (B) ACUTE LYMPHOBLASTIC LEUKEMIA. The Ph-like (also known as BCR-ABL1-like) acute
lymphoblastic leukemia subtype comprises a number of chromosomal translocations and mutations in onco-
genic kinase and cytokine receptor pathways, which are shown in the insert.
of pediatric and adult patients face a much poorer prognosis, and of a wide range of clinical and genetic prognostic markers has allowed
much progress remains to be made. the development of treatment protocols tailored to an individual
patient’s risk of relapse, robust pretreatment prognostic markers
37
had been lacking in T-cell ALL until the recent identification of
T-Cell Acute Lymphoblastic Leukemia biomarkers of differentiation arrest at the earliest stages of T-cell
development. 38,39
Lymphoblasts with a T-cell phenotype comprise approximately Recent work has identified a subset of T-cell ALL cases at particu-
15% of cases of ALL. These cases can be classified according to larly high risk of treatment failure, which are characterized by
the sequence of expression of T-cell–associated surface antigens differentiation arrest at the earliest identifiable stages of T-cell devel-
during normal thymocyte ontogeny. 29,30 Numerous investigators, opment. These cases are defined either by absence of biallelic TCRγ
using a battery of monoclonal antibodies specific for T-cell surface deletion (ABD), indicating differentiation arrest prior to the comple-
glycoproteins, have confirmed the close relationship between the tion of T-cell receptor (TCR) gene rearrangement, or by expression
recognizable patterns of surface antigen expression on leukemic T of a characteristic “early T-cell precursor” (ETP) phenotype. 38,39 In
cells and the normal stages of thymocyte development. 31–34 T-cell ALL addition to harboring oncogenic lesions typical of T-cell ALL, ETP
is often associated with distinctive clinical features that include high cases also harbor gene mutations that are characteristic of AML or
circulating leukocyte counts, a male predominance, CNS involve- myelodysplastic syndrome, including DNMT3A, IDH1/2, and
ment, and a radiographically evident thymic mass in many cases at EZH2, 40,41 suggesting that the cell of origin of ABD/ETP T-cell ALL
presentation. Historically, patients with T-cell ALL had an adverse may be a multipotent hematopoietic stem or progenitor cell rather
prognosis compared with patients with B-lineage ALL, but this gap than a committed T-cell progenitor. ETP appears to comprise
has narrowed with the intensification of therapy for these patients. 35,36 8%–15% of pediatric T-cell ALL but may account for a much higher
41
In marked contrast to precursor B-cell ALL, in which the availability fraction of T-cell ALL in adults, thus providing one possible