Page 1176 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1176
1024 Part VII Hematologic Malignancies
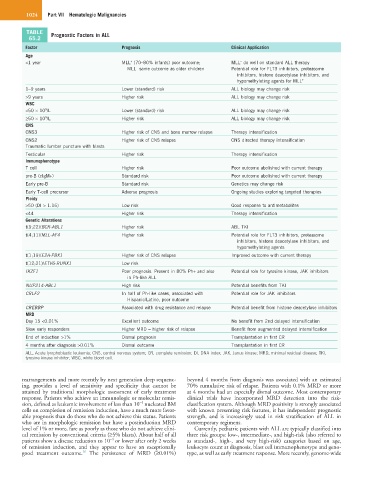
TABLE Prognostic Factors in ALL
65.2
Factor Prognosis Clinical Application
Age
<1 year MLL (70–80% infants) poor outcome; MLL do well on standard ALL therapy
+
−
−
MLL same outcome as older children Potential role for FLT3 inhibitors, proteasome
inhibitors, histone deacetylase inhibitors, and
hypomethylating agents for MLL +
1–9 years Lower (standard) risk ALL biology may change risk
>9 years Higher risk ALL biology may change risk
WBC
9
<50 × 10 /L Lower (standard) risk ALL biology may change risk
≥50 × 10 /L Higher risk ALL biology may change risk
9
CNS
CNS3 Higher risk of CNS and bone marrow relapse Therapy intensification
CNS2 Higher risk of CNS relapse CNS directed therapy intensification
Traumatic lumbar puncture with blasts
Testicular Higher risk Therapy intensification
Immunophenotype
T cell Higher risk Poor outcome abolished with current therapy
pre-B (cIgM+) Standard risk Poor outcome abolished with current therapy
Early pre-B Standard risk Genetics may change risk
Early T-cell precursor Adverse prognosis Ongoing studies exploring targeted therapies
Ploidy
>50 (DI > 1.16) Low risk Good response to antimetabolites
<44 Higher risk Therapy intensification
Genetic Alterations
t(9;22)/BCR-ABL1 Higher risk ABL TKI
t(4;11)/MLL-AF4 Higher risk Potential role for FLT3 inhibitors, proteasome
inhibitors, histone deacetylase inhibitors, and
hypomethylating agents
t(1;19)/E2A-PBX1 Higher risk of CNS relapse Improved outcome with current therapy
t(12;21)/ETV6-RUNX1 Low risk
IKZF1 Poor prognosis. Present in 80% Ph+ and also Potential role for tyrosine kinase, JAK inhibitors
in Ph-like ALL
NUP214-ABL1 High risk Potential benefits from TKI
CRLF2 In half of Ph-like cases, associated with Potential role for JAK inhibitors
Hispanic/Latino, poor outcome
CREBBP Associated with drug resistance and relapse Potential benefit from histone deacetylase inhibitors
MRD
Day 15 <0.01% Excellent outcome No benefit from 2nd delayed intensification
Slow early responders Higher MRD = higher risk of relapse Benefit from augmented delayed intensification
End of induction >1% Dismal prognosis Transplantation in first CR
4 months after diagnosis >0.01% Dismal outcome Transplantation in first CR
ALL, Acute lymphoblastic leukemia; CNS, central nervous system; CR, complete remission; DI, DNA index; JAK, Janus kinase; MRD, minimal residual disease; TKI,
tyrosine kinase inhibitor; WBC, white blood cell.
rearrangements and more recently by next generation deep sequenc- beyond 4 months from diagnosis was associated with an estimated
ing, provides a level of sensitivity and specificity that cannot be 70% cumulative risk of relapse. Patients with 0.1% MRD or more
attained by traditional morphologic assessment of early treatment at 4 months had an especially dismal outcome. Most contemporary
response. Patients who achieve an immunologic or molecular remis- clinical trials have incorporated MRD detection into the risk-
−4
sion, defined as leukemic involvement of less than 10 nucleated BM classification system. Although MRD positivity is strongly associated
cells on completion of remission induction, have a much more favor- with known presenting risk features, it has independent prognostic
able prognosis than do those who do not achieve this status. Patients strength, and is increasingly used in risk stratification of ALL in
who are in morphologic remission but have a postinduction MRD contemporary regimens.
level of 1% or more, fare as poorly as those who do not achieve clini- Currently, pediatric patients with ALL are typically classified into
cal remission by conventional criteria (≥5% blasts). About half of all three risk groups: low-, intermediate-, and high-risk (also referred to
−4
patients show a disease reduction to 10 or lower after only 2 weeks as standard-, high-, and very high-risk) categories based on age,
of remission induction, and they appear to have an exceptionally leukocyte count at diagnosis, blast cell immunophenotype and geno-
16
good treatment outcome. The persistence of MRD (≥0.01%) type, as well as early treatment response. More recently, genome-wide