Page 1227 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1227
Chapter 68 The Polycythemias 1073
VHL protein has other binding partners, including atypical protein suppresses EPO-dependent JAK2–STAT5 signaling. Thus, deletion
kinase C and a family of deubiquitinating enzymes called VHL- of the distal C-terminal cytoplasmic portion of the EPOR abolishes
interacting deubiquitinating enzymes 1 and 2. In addition, VHL has negative regulatory elements and results in increased proliferation of
been involved in numerous cellular processes, including regulation of erythroid progenitor cells. Mutations in the EPOR gene have been
extracellular matrix, cytoskeleton stability, cell cycle control, and observed in some patients with primary familial and congenital
differentiation. VHL disease is not associated with erythrocytosis. polycythemia (PFCP) and are occasionally found in erythroleukemia
(see Fig. 68.2). Such secondary growth factors as insulin-like growth
factor-1 (IGF-1) and the components of the renin–angiotensin
THE ERYTHROPOIETIN RECEPTOR system (RAS) may also influence the production of RBCs.
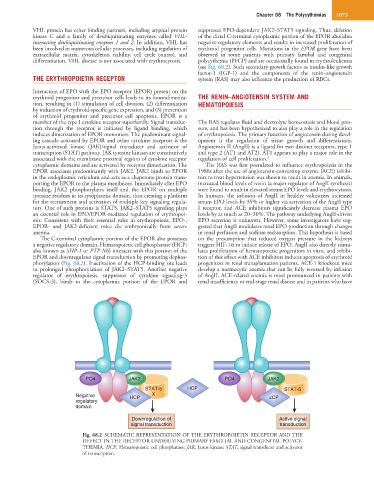
Interaction of EPO with the EPO receptor (EPOR) present on the
erythroid progenitor and precursor cells leads to its homodimeriza- THE RENIN–ANGIOTENSIN SYSTEM AND
tion, resulting in (1) stimulation of cell division, (2) differentiation HEMATOPOIESIS
by induction of erythroid-specific gene expression, and (3) prevention
of erythroid progenitor and precursor cell apoptosis. EPOR is a
member of the type I cytokine receptor superfamily. Signal transduc- The RAS regulates fluid and electrolyte homeostasis and blood pres-
tion through the receptor is initiated by ligand binding, which sure, and has been hypothesized to also play a role in the regulation
induces dimerization of EPOR monomers. The predominant signal- of erythropoiesis. The primary function of angiotensin during devel-
ing cascade activated by EPOR and other cytokine receptors is the opment is the regulation of tissue growth and differentiation.
Janus-activated kinase (JAK)/signal transducer and activator of Angiotensin II (AngII) is a ligand for two distinct receptors, type 1
transcription (STAT) pathway. JAK tyrosine kinases are constitutively and type 2 (AT1 and AT2). AT1 appears to play a major role in the
associated with the membrane proximal regions of cytokine receptor regulation of cell proliferation. 3
cytoplasmic domains and are activated by receptor dimerization. The The RAS was first postulated to influence erythropoiesis in the
EPOR associates predominantly with JAK2. JAK2 binds to EPOR 1980s after the use of angiotensin-converting enzyme (ACE) inhibi-
in the endoplasmic reticulum and acts as a chaperone protein trans- tors to treat hypertension was shown to result in anemia. In animals,
porting the EPOR to the plasma membrane. Immediately after EPO increased blood levels of renin (a major regulator of AngII synthesis)
binding, JAK2 phosphorylates itself and the EPOR on multiple were found to result in elevated serum EPO levels and erythrocytosis.
tyrosine residues in its cytoplasmic domain, thus creating a platform In humans, the infusion of AngII in healthy volunteers increased
for the recruitment and activation of multiple key signaling regula- serum EPO levels by 35% or higher via activation of the AngII type
tors. One of such proteins is STAT5. JAK2–STAT5 signaling plays I receptor, and ACE inhibitors significantly decrease plasma EPO
an essential role in EPO/EPOR-mediated regulation of erythropoi- levels by as much as 20–30%. The pathway underlying AngII-driven
esis. Consistent with their essential roles in erythropoiesis, EPO-, EPO secretion is unknown. However, some investigators have sug-
EPOR- and JAK2-deficient mice die embryonically from severe gested that AngII modulates renal EPO production through changes
anemia. in renal perfusion and sodium reabsorption. This hypothesis is based
The C-terminal cytoplasmic portion of the EPOR also possesses on the presumption that reduced oxygen pressure in the kidneys
a negative regulatory domain. Hematopoietic cell phosphatase (HCP; triggers HIF-1α to induce release of EPO. AngII also directly stimu-
also known as SHP-1 or PTP N6) interacts with this portion of the lates proliferation of hematopoietic progenitors in vitro, and inhibi-
EPOR and downregulates signal transduction by promoting dephos- tion of this effect with ACE inhibitors induces apoptosis of erythroid
phorylation (Fig. 68.2). Inactivation of the HCP-binding site leads progenitors in renal transplantation patients. ACE-1 knockout mice
to prolonged phosphorylation of JAK2–STAT5. Another negative develop a normocytic anemia that can be fully reversed by infusion
regulator of erythropoiesis, suppressor of cytokine signaling-3 of AngII. ACE-related anemia is most pronounced in patients with
(SOCS-3), binds to the cytoplasmic portion of the EPOR and renal insufficiency or end-stage renal disease and in patients who have
PO4 JAK2 PO4 JAK2
STAT-5 HCP STAT-5
Negative HCP X κCP
regulatory
domain
Downregulation of Active signal
signal transduction transduction
Fig. 68.2 SCHEMATIC REPRESENTATION OF THE ERYTHROPOIETIN RECEPTOR AND THE
DEFECT IN THE RECEPTOR UNDERLYING PRIMARY FAMILIAL AND CONGENITAL POLYCY-
THEMIA. HCP, Hematopoietic cell phosphatase; JAK, Janus kinase; STAT, signal transducer and activator
of transcription.