Page 125 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 125
96 Part II Cellular Basis of Hematology
Mouse
LT-HSC ST-HSC MPP
–
–
–
–
lo
+
+
+
c-Kit + Thy-1.1 lo Lin Sca-1 + c-Kit Thy-1.1 Lin Sca-1 c-Kit Thy-1.1 Lin Sca-1 +
–
–
–
+
+
FLK2 CD34 CD150 + FLK2 CD34 + CD150 + FLK2 CD34 CD150 –
Human
LT-HSC ST-HSC MPP
–
–
–
–
+
+
CD34 CD38 Lin CD34 CD38 Lin
–
–
–
–
+
+
CD45RA Thy1 CD49f CD45RA Thy1 CD49f
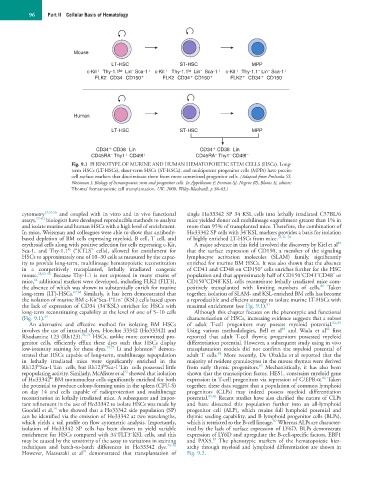
Fig. 9.1 PHENOTYPE OF MURINE AND HUMAN HEMATOPOIETIC STEM CELLS (HSCs). Long-
term HSCs (LT-HSCs), short-term HSCs (ST-HSCs), and multipotent progenitor cells (MPPs) have precise
cell surface markers that discriminate them from more committed progenitor cells. (Adapted from Prohaska SS,
Weissman I: Biology of hematopoietic stem and progenitor cells. In Appelbaum F, Forman SJ, Negrin RS, Blume K, editors:
Thomas’ hematopoietic cell transplantation, UK, 2008, Wiley-Blackwell, p 36-63.)
−
cytometry 17,55,56 and coupled with in vitro and in vivo functional single Ho33342 SP 34 KSL cells into lethally irradiated C57BL/6
assays, 57–62 biologists have developed reproducible methods to analyze mice yielded donor cell multilineage engraftment greater than 1% in
and isolate murine and human HSCs with a high level of enrichment. more than 95% of transplanted mice. Therefore, the combination of
−
In mice, Weissman and colleagues were able to show that antibody- Ho33342 SP cells with 34 KSL markers provides a basis for isolation
based depletion of BM cells expressing myeloid, B cell, T cell, and of highly enriched LT-HSCs from mice. 55,76–78
80
erythroid cells along with positive selection for cells expressing c-Kit, A major advance in this field involved the discovery by Kiel et al
lo
Sca-1, and Thy-1.1 (“KTLS” cells), allowed for enrichment for that the surface expression of CD150, a member of the signaling
HSCs to approximately one of 10–30 cells as measured by the capac- lymphocyte activation molecules (SLAM) family, significantly
ity to provide long-term, multilineage hematopoietic reconstitution enriched for murine BM HSCs. It was also shown that the absence
+
in a competitively transplanted, lethally irradiated congenic of CD41 and CD48 on CD150 cells enriches further for the HSC
+
−
−
mouse. 58,63–66 Because Thy-1.1 is not expressed in many strains of population and that approximately half of CD150 CD41 CD48 or
−
+
64
mice, additional markers were developed, including FLK2 (FLT3), CD150 CD48 KSL cells reconstitute lethally irradiated mice com-
80
the absence of which was shown to substantially enrich for murine petitively transplanted with limiting numbers of cells. Taken
long-term (LT)-HSCs. 67,68 Similarly, it has been demonstrated that together, isolation of SLAM- and KSL-enriched BM cells has become
−
+
+
the isolation of murine BM c-Kit Sca-1 Lin (KSL) cells based upon a reproducible and efficient strategy to isolate murine LT-HSCs with
−
the lack of expression of CD34 (34 KSL) enriches for HSCs with maximal enrichment (see Fig. 9.1). 81
long-term reconstituting capability at the level of one of 5–10 cells Although this chapter focuses on the phenotypic and functional
(Fig. 9.1). 69 characterization of HSCs, increasing evidence suggests that a subset
An alternative and effective method for isolating BM HSCs of adult T-cell progenitors may possess myeloid potential. 82–84
82
83
involves the use of intravital dyes, Hoechst 33342 (Ho33342) and Using various methodologies, Bell et al and Wada et al first
Rhodamine 123 (Rh123). 70–74 HSCs, unlike more committed pro- reported that adult T-cell thymic progenitors possessed myeloid
genitor cells, efficiently efflux these dyes such that HSCs display differentiation potential. However, a subsequent study using in vivo
73
low-intensity staining for these dyes. 74,75 Li and Johnson demon- transplantation models did not confirm the myeloid potential of
84
strated that HSCs capable of long-term, multilineage repopulation adult T cells. More recently, De Obaldia et al reported that the
in lethally irradiated mice were significantly enriched in the majority of resident granulocytes in the mouse thymus were derived
+
−
−
+
hi
85
lo
Rh123 Sca-1 Lin cells, but Rh123 Sca-1 Lin cells possessed little from early thymic progenitors. Mechanistically, it has also been
72
repopulating activity. Similarly, McAlister et al showed that isolation shown that the transcription factor, HES1, constrains myeloid gene
lo
86
of Ho33342 BM mononuclear cells significantly enriched for both expression in T-cell progenitors via repression of C/EPB-α. Taken
the potential to produce colony-forming units in the spleen (CFU-S) together, these data suggest that a population of common lymphoid
on day 14 and cells capable of radioprotection and multilineage progenitors (CLPs) may indeed possess myeloid differentiation
reconstitution in lethally irradiated mice. A subsequent and impor- potential. 85,86 Recent studies have also clarified the nature of CLPs
tant refinement in the use of Ho33342 to isolate HSCs was made by and have dissected this population further into an all-lymphoid
74
Goodell et al, who showed that a Ho33342 side population (SP) progenitor cell (ALP), which retains full lymphoid potential and
can be identified via the emission of Ho33342 at two wavelengths, thymic seeding capability, and B lymphoid progenitor cells (BLPs),
87
which yields a tail profile on flow cytometric analysis. Importantly, which is restricted to the B-cell lineage. Whereas ALPs are character-
isolation of Ho33342 SP cells has been shown to yield variable ized by the lack of surface expression of LY6D, BLPs demonstrate
−
−
enrichment for HSCs compared with 34 FLT3 KSL cells, and this expression of LY6D and upregulate the B-cell-specific factors, EBF1
87
may be caused by the sensitivity of the assay to variations in staining and PAX5. The phenotypic markers of the hematopoietic hier-
techniques and batch-to-batch differences in Ho33342 dye. 76–78 archy through myeloid and lymphoid differentiation are shown in
79
However, Matsuzaki et al demonstrated that transplantation of Fig. 9.2.