Page 1508 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1508
1340 Part VII Hematologic Malignancies
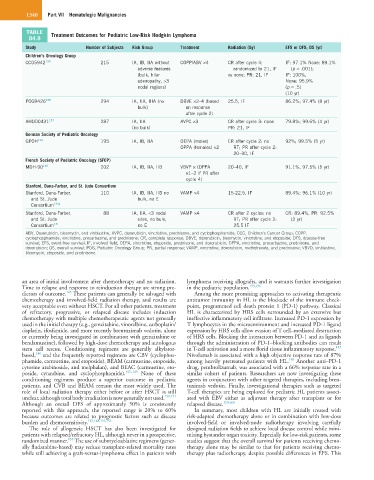
TABLE Treatment Outcomes for Pediatric Low-Risk Hodgkin Lymphoma
84.8
Study Number of Subjects Risk Group Treatment Radiation (Gy) EFS or DFS; OS (yr)
Children’s Oncology Group
CCG5942 139 215 IA, IB, IIA without COPP/ABV ×4 CR after cycle 4: IF: 97.1% None: 89.1%
adverse features randomized to 21, IF (p = .001);
(bulk, hilar vs none; PR: 21, IF IF: 100%,
adenopathy, >3 None: 95.9%
nodal regions) (p = .5)
(10 yr)
POG9426 138 294 IA, IIA, IIIA (no DBVE ×2–4 (based 25.5, IF 86.2%; 97.4% (8 yr)
bulk) on response
after cycle 2)
AHOD0431 137 287 IA, IIA AVPC ×3 CR after cycle 3: none 79.8%; 99.6% (4 yr)
(no bulk) PR: 21, IF
German Society of Pediatric Oncology
GPOH 134 195 IA, IB, IIA OEPA (males) CR after cycle 2: no 92%; 99.5% (5 yr)
OPPA (females) ×2 RT; PR after cycle 2:
20–30, IF
French Society of Pediatric Oncology (SFOP)
MDH-90 140 202 IA, IB, IIA, IIB VBVP × (OPPA 20–40, IF 91.1%, 97.5% (5 yr)
×1–2 if PR after
cycle 4)
Stanford, Dana-Farber, and St. Jude Consortium
Stanford, Dana-Farber, 110 IA, IB, IIA, IIB no VAMP ×4 15-22.5, IF 89.4%; 96.1% (10 yr)
and St. Jude bulk, no E
Consortium 144a
Stanford, Dana-Farber, 88 IA, IIA, <3 nodal VAMP ×4 CR after 2 cycles: no CR: 89.4%, PR: 92.5%
and St. Jude sites, no bulk, RT; PR after cycle 2: (2 yr)
Consortium 141 no E 25.5 IF
ABV, Doxorubicin, bleomycin, and vinblastine; AVPC, doxorubicin, vincristine, prednisone, and cyclophosphamide; CCG, Children’s Cancer Group; COPP,
cyclophosphamide, vincristine, procarbazine, and prednisone; CR, complete response; DBVE, doxorubicin, bleomycin, vincristine, and etoposide; DFS, disease-free
survival; EFS, event-free survival; IF, involved field; OEPA, vincristine, etoposide, prednisone, and doxorubicin; OPPA, vincristine, procarbazine, prednisone, and
doxorubicin; OS, overall survival; POG, Pediatric Oncology Group; PR, partial response; VAMP, vincristine, doxorubicin, methotrexate, and prednisone; VBVD, vinblastine,
bleomycin, etoposide, and prednisone.
an area of initial involvement after chemotherapy and no radiation. lymphoma receiving allografts, and it warrants further investigation
Time to relapse and response to reinduction therapy are strong pre- in the pediatric population. 155,156
145
dictors of outcome. These patients can generally be salvaged with Among the more promising approaches to activating therapeutic
chemotherapy and involved-field radiation therapy, and results are antitumor immunity in HL is the blockade of the immune check-
very acceptable even without HSCT. For all other patients, treatment point, programmed cell death protein 1 (PD-1) pathway. Classical
of refractory, progressive, or relapsed disease includes induction HL is characterized by HRS cells surrounded by an extensive but
chemotherapy with multiple chemotherapeutic agents not generally ineffective inflammatory cell infiltrate. Increased PD-1 expression by
used in the initial therapy (e.g., gemcitabine, vinorelbine, carboplatin/ T lymphocytes in the microenvironment and increased PD-1 ligand
cisplatin, ifosfamide, and more recently brentuximab vedotin, alone expression by HRS cells allow evasion of T cell–mediated destruction
or currently being investigated in combination with gemcitabine or of HRS cells. Blocking the interaction between PD-1 and its ligands
bendamustine), followed by high-dose chemotherapy and autologous through the administration of PD-1–blocking antibodies can result
157
stem cell rescue. Conditioning regimens are generally alkylator- in T-cell activation and a more florid tissue inflammatory response.
146
based, and the frequently reported regimens are CBV (cyclophos- Nivolumab is associated with a high objective response rate of 87%
158
phamide, carmustine, and etoposide), BEAM (carmustine, etoposide, among heavily pretreated patients with HL. Another anti–PD-1
cytosine arabinoside, and melphalan), and BEAC (carmustine, eto- drug, pembrolizumab, was associated with a 66% response rate in a
poside, cytarabine, and cyclophosphamide). 147–149 None of these similar cohort of patients. Researchers are now investigating these
conditioning regimens produce a superior outcome in pediatric agents in conjunction with other targeted therapies, including bren-
patients, and CVB and BEAM remain the most widely used. The tuximab vedotin. Finally, investigational therapies such as targeted
role of local radiation therapy either before or after HSCT is still T-cell therapies are being explored for pediatric HL patients associ-
unclear, although total body irradiation is now generally not used. 150,151 ated with EBV either as adjuvant therapy after transplant or for
Although an overall DFS of approximately 50% is consistently relapsed disease. 159,160
reported with this approach, the reported range is 20% to 60% In summary, most children with HL are initially treated with
because outcomes are related to prognostic factors such as disease risk-adapted chemotherapy alone or in combination with low-dose
burden and chemosensitivity. 147,148,152,153 involved-field or involved-node radiotherapy involving carefully
The role of allogeneic HSCT has also been investigated for designed radiation fields to achieve local disease control while mini-
patients with relapsed/refractory HL, although never in a prospective, mizing bystander organ toxicity. Especially for low-risk patients, some
154
randomized manner. The use of submyeloablative regimens (gener- studies suggest that the overall survival for patients receiving chemo-
ally fludarabine-based) may reduce transplant-related mortality rates therapy alone may be similar to that for patients receiving chemo-
while still achieving a graft-versus-lymphoma effect in patients with therapy plus radiotherapy, despite possible differences in EFS. This