Page 1505 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1505
Chapter 84 Malignant Lymphomas in Childhood 1337
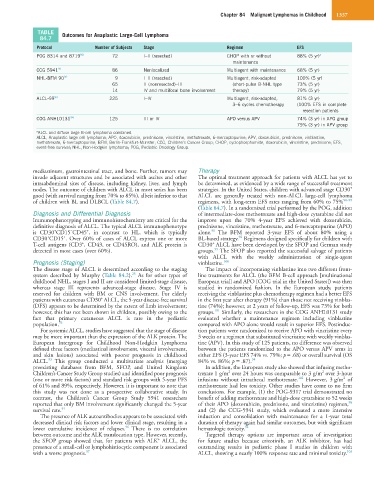
TABLE Outcomes for Anaplastic Large-Cell Lymphoma
84.7
Protocol Number of Subjects Stage Regimen EFS
POG 8314 and 8719 90 72 I–II (resected) CHOP with or without 88% (5 yr) a
maintenance
CCG 5941 91 86 Nonlocalized Multiagent with maintenance 68% (5 yr)
NHL-BFM 90 92 9 I–II (resected) Multiagent, risk-adapted 100% (5 yr)
65 II (nonresected)–III (short-pulse B-NHL type 73% (5 yr)
14 IV and multifocal bone involvement therapy) 79% (5 yr)
ALCL-99 93 225 I–IV Multiagent, risk-adapted, 81% (3 yr)
3–6 cycles chemotherapy (100% EFS in complete
resection patients
COG ANHL0131 94 125 III or IV APO versus APV 74% (3 yr) in APO group
79% (3 yr) in APV group
a ALCL and diffuse large B-cell lymphoma combined.
ALCL, Anaplastic large cell lymphoma; APO, doxorubicin, prednisone, vincristine, methotrexate, 6-mercaptopurine; APV, doxorubicin, prednisone, vinblastine,
methotrexate, 6-mercaptopurine; BFM, Berlin-Frankfurt-Münster; CCG, Children’s Cancer Group; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; EFS,
event-free survival; NHL, Non-Hodgkin lymphoma; POG, Pediatric Oncology Group.
mediastinum, gastrointestinal tract, and bone. Further, tumors may Therapy
invade adjacent structures and be associated with ascites and other The optimal treatment approach for patients with ALCL has yet to
intraabdominal sites of disease, including kidney, liver, and lymph be determined, as evidenced by a wide range of successful treatment
+
nodes. The outcome of children with ALCL in most series has been strategies. In the United States, children with advanced stage CD30
good (with survival ranging from 70% to 85%), albeit inferior to that ALCL are generally treated with non-ALCL large-cell lymphoma
of children with BL and DLBCL (Table 84.7). regimens, with long-term EFS rates ranging from 60% to 75% 90–93
(Table 84.7). In a randomized trial performed by the POG, addition
Diagnosis and Differential Diagnosis of intermediate-dose methotrexate and high-dose cytarabine did not
Immunophenotyping and immunohistochemistry are critical for the improve upon the 70% 4-year EFS achieved with doxorubicin,
definitive diagnosis of ALCL. The typical ALCL immunophenotype prednisone, vincristine, methotrexate, and 6-mercaptopurine (APO)
−
+
+
98
is CD30 CD15 CD45 , in contrast to HL, which is typically alone. The BFM reported 3-year EFS of about 80% using a
+
+
92
CD30 CD15 . Over 60% of cases of ALCL express one or more BL-based strategy. Regimens designed specifically for children with
+
+
T-cell antigens (CD3 , CD43, or CD45RO), and ALK protein is CD30 ALCL have been developed by the SFOP and German study
99
detected in most cases (over 60%). groups. The SFOP also reported the successful salvage of patients
with ALCL with the weekly administration of single-agent
Prognosis (Staging) vinblastine. 100
The disease stage of ALCL is determined according to the staging The impact of incorporating vinblastine into two different front-
25
system described by Murphy (Table 84.2). As for other types of line treatments for ALCL (the BFM B-cell approach [multinational
childhood NHL, stages I and II are considered limited-stage disease, European trial] and APO [COG trial in the United States]) was then
whereas stage III represents advanced-stage disease. Stage IV is studied in randomized fashion. In the European study, patients
reserved for children with BM or CNS involvement. For elderly receiving the vinblastine plus chemotherapy regimen had a better EFS
+
patients with cutaneous CD30 ALCL, the 5-year disease-free survival in the first year after therapy (91%) than those not receiving vinblas-
(DFS) appears to be determined by the extent of limb involvement; tine (74%); however, at 2 years of follow-up, EFS was 73% for both
101
however, this has not been shown in children, possibly owing to the groups. Similarly, the researchers in the COG ANHL0131 study
fact that primary cutaneous ALCL is rare in the pediatric evaluated whether a maintenance regimen including vinblastine
population. 95 compared with APO alone would result in superior EFS. Postinduc-
For systemic ALCL, studies have suggested that the stage of disease tion patients were randomized to receive APO with vincristine every
may be more important than the expression of the ALK protein. The 3 weeks or a regimen that substituted vincristine with weekly vinblas-
European Intergroup for Childhood Non-Hodgkin Lymphoma tine (APV). In this study of 125 patients, no difference was observed
defined three factors (mediastinal involvement, visceral involvement, between the patients randomized to the APO versus APV arms in
and skin lesions) associated with poorer prognosis in childhood either EFS (3-year EFS 74% vs. 79%; p = .68) or overall survival (OS
93
ALCL. This group conducted a multivariate analysis (merging 84% vs. 86%; p = .87). 94
preexisting databases from BFM, SFOP, and United Kingdom In addition, the European study also showed that infusing metho-
2
2
Children’s Cancer Study Group studies) and identified poor prognosis trexate 1 g/m over 24 hours was comparable to 3 g/m over 3-hour
102
2
(one or more risk factors) and standard risk groups with 5-year PFS infusions without intrathecal methotrexate. However, 3 g/m of
of 61% and 89%, respectively. However, it is important to note that methotrexate had less toxicity. Other studies have come to no firm
this study was not done as a prospective collaborative study. In conclusions. For example, (1) the POG-9317 trial demonstrated no
contrast, the Children’s Cancer Group Study 5941 researchers benefit of adding methotrexate and high-dose cytarabine to 52 weeks
98
reported that only BM involvement significantly changed the 5-year of their APO (doxorubicin, prednisone, and vincristine) regimen,
survival rate. 91 and (2) the CCG-5941 study, which evaluated a more intensive
The presence of ALK autoantibodies appears to be associated with induction and consolidation with maintenance for a 1-year total
decreased clinical risk factors and lower clinical stage, resulting in a duration of therapy again had similar outcomes, but with significant
96
lower cumulative incidence of relapses. There is no correlation hematologic toxicity. 92
between outcome and the ALK translocation type. However, recently, Targeted therapy options are important areas of investigation
+
the SFOP group showed that, for patients with ALK ALCL, the for future studies because crizotinib, an ALK inhibitor, has had
presence of a small-cell or lymphohistiocytic component is associated outstanding results in pediatric phase I studies in children with
103
with a worse prognosis. 97 ALCL, showing a nearly 100% response rate and minimal toxicity.