Page 1710 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1710
1520 Part IX Cell-Based Therapies
C
A
D
B
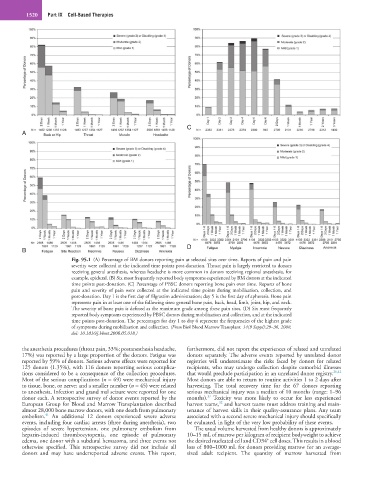
Fig. 95.1 (A) Percentage of BM donors reporting pain at selected sites over time. Reports of pain and pain
severity were collected at the indicated time points post-donation. Throat pain is largely restricted to donors
receiving general anesthesia, whereas headache is more common in donors receiving regional anesthesia, for
example, epidural. (B) Six most frequently reported body symptoms experienced by BM donors at the indicated
time points post-donation. (C) Percentage of PBSC donors reporting bone pain over time. Reports of bone
pain and severity of pain were collected at the indicated time points during mobilization, collection, and
post-donation. Day 1 is the first day of filgrastim administration; day 5 is the first day of apheresis. Bone pain
represents pain in at least one of the following sites: general bone pain, back, head, limb, joint, hip, and neck.
The severity of bone pain is defined as the maximum grade among these pain sites. (D) Six most frequently
reported body symptoms experienced by PBSC donors during mobilization and collection, and at the indicated
time points post-donation. The percentages for day 1 to day 6 represent the frequencies of the highest grade
of symptoms during mobilization and collection. (From Biol Blood Marrow Transplant. 14(9 Suppl):29–36, 2008,
doi: 10.1016/j.bbmt.2008.05.018.)
the anesthesia procedures (throat pain, 33%; postanesthesia headache, furthermore, did not report the experiences of related and unrelated
17%) was reported by a large proportion of the donors. Fatigue was donors separately. The adverse events reported by unrelated donor
reported by 59% of donors. Serious adverse effects were reported for registries will underestimate the risks faced by donors for related
125 donors (1.35%), with 116 donors reporting serious complica- recipients, who may undergo collection despite comorbid illnesses
tions considered to be a consequence of the collection procedures. that would preclude participation in an unrelated donor registry. 26,42
Most of the serious complications (n = 69) were mechanical injury Most donors are able to return to routine activities 1 to 2 days after
to tissue, bone, or nerve; and a smaller number (n = 45) were related harvesting, The total recovery time for the 67 donors reporting
to anesthesia. Infection and grand mal seizure were reported for one serious mechanical injury was a median of 10 months (range: 1–96
37
donor each. A retrospective survey of donor events reported by the months). Toxicity was more likely to occur for less experienced
32
European Group for Blood and Marrow Transplantation described harvest teams, and harvest teams must address training and main-
almost 28,000 bone marrow donors, with one death from pulmonary tenance of harvest skills in their quality-assurance plans. Any team
41
embolism. An additional 12 donors experienced severe adverse associated with a second severe mechanical injury should specifically
events, including four cardiac arrests (three during anesthesia), two be evaluated, in light of the very low probability of these events.
episodes of severe hypertension, one pulmonary embolism from The usual volume harvested from healthy donors is approximately
heparin-induced thrombocytopenia, one episode of pulmonary 10–15 mL of marrow per kilogram of recipient bodyweight to achieve
+
edema, one donor with a subdural hematoma, and three events not the desired nucleated cell and CD34 cell doses. This results in a blood
otherwise specified. This retrospective survey did not include all loss of 800–1000 mL for donors providing marrow for an average-
donors and may have underreported adverse events. This report, sized adult recipient. The quantity of marrow harvested from