Page 1766 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1766
1570 Part IX Cell-Based Therapies
Chimeric antigen receptor
αβTCR
Redirecting T cell
specificity
Costimulation
Autocrine production of IL-2
production of cytokines IL-15
T-GFβ
Tumor cells
FasL
IL-7
Fas
Reduced sensitivity to FasL
IL−7Rα mediated apoptosis
Restore the response BCL-2
homeostatic cytokines Chemokine
Overexpression of
Expression of the antiapoptotic molecules
specific chemokine
receptor
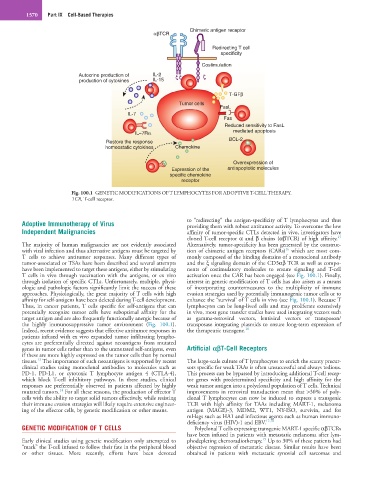
Fig. 100.1 GENETIC MODIFICATIONS OF T LYMPHOCYTES FOR ADOPTIVE T-CELL THERAPY.
TCR, T-cell receptor.
Adoptive Immunotherapy of Virus to “redirecting” the antigen-specificity of T lymphocytes and thus
providing them with robust antitumor activity. To overcome the low
Independent Malignancies affinity of tumor-specific CTLs detected in vivo, investigators have
15
cloned T-cell receptor α and β chains (αβTCR) of high affinity.
The majority of human malignancies are not evidently associated Alternatively, tumor-specificity has been generated by the construc-
16
with viral infection and thus alternative antigens must be targeted by tion of chimeric antigen receptors (CARs) which are most com-
T cells to achieve antitumor responses. Many different types of monly composed of the binding domains of a monoclonal antibody
tumor-associated or TSAs have been described and several attempts and the ξ signaling domain of the CD3αβ TCR as well as compo-
have been implemented to target these antigens, either by stimulating nents of costimulatory molecules to ensure signaling and T-cell
T cells in vivo through vaccination with the antigens, or ex vivo activation once the CAR has been engaged (see Fig. 100.1). Finally,
through isolation of specific CTLs. Unfortunately, multiple, physi- interest in genetic modification of T cells has also arisen as a means
ologic and pathologic factors significantly limit the success of these of incorporating countermeasures to the multiplicity of immune
approaches. Physiologically, the great majority of T cells with high evasion strategies used by potentially immunogenic tumor cells or to
affinity for self-antigens have been deleted during T-cell development. enhance the “survival’ of T cells in vivo (see Fig. 100.1). Because T
Thus, in cancer patients, T cells specific for self-antigens that can lymphocytes can be long-lived cells and may proliferate extensively
potentially recognize tumor cells have suboptimal affinity for the in vivo, most gene transfer studies have used integrating vectors such
target antigen and are also frequently functionally anergic because of as gamma-retroviral vectors, lentiviral vectors or transposon/
the highly immunosuppressive tumor environment (Fig. 100.1). transposase integrating plasmids to ensure long-term expression of
Indeed, recent evidence suggests that effective antitumor responses in the therapeutic transgene. 16
patients infused with ex vivo expanded tumor infiltrating lympho-
cytes are preferentially directed against neoantigens from mutated
genes in tumor cells rather than to the unmutated self-antigens, even Artificial αβT-Cell Receptors
if these are more highly expressed on the tumor cells than by normal
13
tissues. The importance of such neoantigens is supported by recent The large-scale culture of T lymphocytes to enrich the scanty precur-
clinical studies using monoclonal antibodies to molecules such as sors specific for weak TAAs is often unsuccessful and always tedious.
PD-1, PD-L1, or cytotoxic T lymphocyte antigen 4 (CTLA-4), This process can be bypassed by introducing additional T-cell recep-
which block T-cell inhibitory pathways. In these studies, clinical tor genes with predetermined specificity and high affinity for the
responses are preferentially observed in patients affected by highly weak tumor antigen into a polyclonal population of T cells. Technical
14
mutated tumors. For all these reasons, the production of effector T improvements in retroviral transduction mean that >30% of poly-
cells with the ability to target solid tumors effectively, while resisting clonal T lymphocytes can now be induced to express a transgenic
their immune evasion strategies will likely require extensive engineer- TCR with high affinity for TAAs including MART-1, melanoma
ing of the effector cells, by genetic modification or other means. antigen (MAGE)-3, MDM2, WT1, NY-ISO, survivin, and for
mHags such as HA1 and infectious agents such as human immuno-
deficiency virus (HIV)-1 and EBV. 17,18
GENETIC MODIFICATION OF T CELLS Polyclonal T cells expressing transgenic MART-1 specific αβTCRs
have been infused in patients with metastatic melanoma after lym-
15
Early clinical studies using genetic modification only attempted to phodepleting chemoradiotherapy. Up to 30% of these patients had
“mark” the T-cell infused to follow their fate in the peripheral blood objective regression of metastatic disease. Similar results have been
or other tissues. More recently, efforts have been devoted obtained in patients with metastatic synovial cell sarcomas and