Page 1768 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1768
1572 Part IX Cell-Based Therapies
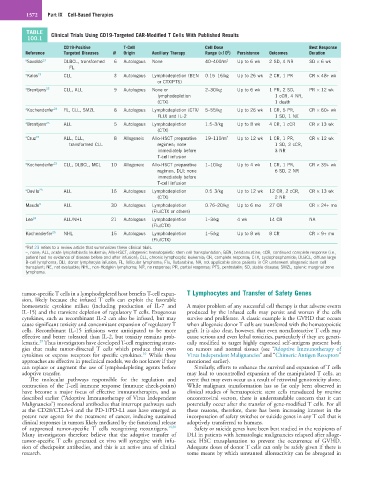
TABLE Clinical Trials Using CD19-Targeted CAR-Modified T Cells With Published Results
100.1
CD19-Positive T-Cell Cell Dose Best Response
Reference Targeted Diseases N Origin Auxiliary Therapy Range (×10 ) Persistence Outcomes Duration
6
a Savoldo 23 DLBCL, transformed 6 Autologous None 40–400/m 2 Up to 6 wk 2 SD, 4 NR SD × 6 wk
FL
a Kalos 23 CLL 3 Autologous Lymphodepletion (BEN 0.15–16/kg Up to 26 wk 2 CR, 1 PR CR × 48+ wk
or CTX/PTS)
a Brentjens 23 CLL, ALL 9 Autologous None or 2–30/kg Up to 6 wk 1 PR, 2 SD, PR × 12 wk
lymphodepletion 1 cCR, 4 NR,
(CTX) 1 death
a Kochenderfer 23 FL, CLL, SMZL 8 Autologous Lymphodepletion (CTX/ 5–55/kg Up to 26 wk 1 CR, 5 PR, CR × 60+ wk
FLU) and IL-2 1 SD, 1 NE
a Brentjens 23 ALL 5 Autologous Lymphodepletion 1.5–3/kg Up to 8 wk 4 CR, 1 cCR CR × 13 wk
(CTX)
a Cruz 23 ALL, CLL, 8 Allogeneic Allo-HSCT preparative 19–110/m 2 Up to 12 wk 1 CR, 1 PR, CR × 12 wk
transformed CLL regimen; none 1 SD, 2 cCR,
immediately before 3 NR
T-cell infusion
a Kochenderfer 23 CLL, DLBCL, MCL 10 Allogeneic Allo-HSCT preparative 1–10/kg Up to 4 wk 1 CR, 1 PR, CR × 39+ wk
regimen, DLI; none 6 SD, 2 NR
immediately before
T-cell infusion
a Davila 23 ALL 16 Autologous Lymphodepletion 0.5–3/kg Up to 12 wk 12 CR, 2 cCR, CR × 13 wk
(CTX) 2 NR
Maude 3 ALL 30 Autologous Lymphodepletion 0.76–20/kg Up to 6 mo 27 CR CR × 24+ mo
(Flu/CTX or others)
Lee 24 ALL/NHL 21 Autologous Lymphodepletion 1–3/kg 4 wk 14 CR NA
(Flu/CTX)
Kochenderfer 25 NHL 15 Autologous Lymphodepletion 1–5/kg Up to 8 wk 8 CR CR × 9+ mo
(Flu/CTX)
a Ref 23 refers to a review article that summarizes these clinical trials.
–, none; ALL, acute lymphoblastic leukemia; Allo-HSCT, allogeneic hematopoietic stem cell transplantation; BEN, bendamustine; cCR, continued complete response (i.e.,
patient had no evidence of disease before and after infusion); CLL, chronic lymphocytic leukemia; CR, complete response; CTX, cyclophosphamide; DLBCL, diffuse large
B-cell lymphoma; DLI, donor lymphocyte infusion; FL, follicular lymphoma; Flu, fludarabine; NA, not applicable since patients in CR underwent allogeneic stem cell
transplant; NE, not evaluable; NHL, non-Hodgkin lymphoma; NR, no response; PR, partial response; PTS, pentostatin; SD, stable disease; SMZL, splenic marginal zone
lymphoma.
tumor-specific T cells in a lymphodepleted host benefits T-cell expan- T Lymphocytes and Transfer of Safety Genes
sion, likely because the infused T cells can exploit the favorable
homeostatic cytokine milieu (including production of IL-7 and A major problem of any successful cell therapy is that adverse events
IL-15) and the transient depletion of regulatory T cells. Exogenous produced by the infused cells may persist and worsen if the cells
cytokines, such as recombinant IL-2 can also be infused, but may survive and proliferate. A classic example is the GVHD that occurs
cause significant toxicity and concomitant expansion of regulatory T when allogeneic donor T cells are transferred with the hematopoietic
cells. Recombinant IL-15 infusions were anticipated to be more graft. It is also clear, however, that even nonalloreactive T cells may
effective and better tolerated than IL-2, but toxicity remains prob- cause serious and even lethal toxicities, particularly if they are geneti-
29
lematic. Thus investigators have developed T-cell engineering strate- cally modified to target highly expressed self-antigens present both
gies that make tumor-directed T cells which produce their own on tumors and normal tissues (see “Adoptive Immunotherapy of
16
cytokines or express receptors for specific cytokines. While these Virus Independent Malignancies” and “Chimeric Antigen Receptors”
approaches are effective in preclinical models, we do not know if they mentioned earlier).
can replace or augment the use of lymphodepleting agents before Similarly, efforts to enhance the survival and expansion of T cells
adoptive transfer. may lead to uncontrolled expansion of the manipulated T cells, an
The molecular pathways responsible for the regulation and event that may even occur as a result of retroviral genotoxicity alone.
contraction of the T-cell immune response (immune check-points) While malignant transformation has so far only been observed in
have become a major focus of effective immunotherapies, and as clinical studies of hematopoietic stem cells transduced by murine
described earlier (“Adoptive Immunotherapy of Virus Independent oncoretroviral vectors, there is understandable concern that it can
Malignancies”) monoclonal antibodies that interrupt pathways such potentially occur after the transfer of gene-modified T cells. For all
as the CD28/CTLA-4 and the PD-1/PD-L1 axes have emerged as these reasons, therefore, there has been increasing interest in the
potent new agents for the treatment of cancer, inducing sustained incorporation of safety switches or suicide genes in any T cell that is
clinical responses in tumors likely mediated by the functional release adoptively transferred to humans.
of suppressed tumor-specific T cells recognizing neoantigens. 14,30 Safety or suicide genes have been best studied in the recipients of
Many investigators therefore believe that the adoptive transfer of DLI in patients with hematologic malignancies relapsed after alloge-
tumor-specific T cells generated ex vivo will synergize with infu- neic HSC transplantation to prevent the occurrence of GVHD.
sion of checkpoint antibodies, and this is an active area of clinical Adequate doses of donor T cells can only be safely given if there is
research. some means by which unwanted alloreactivity can be abrogated in