Page 1818 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1818
1620 Part X Transplantation
A current consensus, the “missing self” hypothesis, 21,22 is that NK NK cells can attune themselves to the new HLA environment,
cells have evolved to detect and rapidly eliminate virally infected or indicating that NK cell alloreactivity is a complex and dynamic
29
tumor cells that have downregulated cell surface expression of MHC phenomenon. Because the genes comprising the LRC and the HLA
+
class I molecules to evade the CD8 T-cell arm of the immune locus are on chromosomes 19 and 6, respectively, KIR and HLA
response. molecules are inherited independently, so individuals can inherit an
The molecular basis of NK cell alloreactivity is incompletely iKIR but not its ligand, resulting in an NK cell that cannot be
understood, but it involves a dynamic balance of signals through licensed during development. Unlicensed NK cells would be pre-
activating as well as inhibitory receptors on the NK cell. The killer dicted to be poorly functional; unexpectedly, however, they dominate
30
immunoglobulin-like receptors (KIRs) are encoded by a set of linked the early response to cytomegalovirus (CMV) infection in mice, and
genes called the leukocyte receptor complex (LRC) on human chro- they also may be potent mediators of antibody-dependent cellular
23
31
mosome 19q13.4. KIRs contain two or three extracellular Ig-like cytotoxicity. DNA damage resulting from transplant conditioning
domains and either a short (S) or a long (L) cytoplasmic tail, which or inflammatory conditions such as viral infection may be sufficient
32
mediates activating or inhibitory signals, respectively. The LRC is to induce stress ligands of NK cell activation receptors, break toler-
marked by significant interindividual variation in KIR gene content ance in unlicensed NK cells, and generate autoreactivity, 33–35 includ-
24
as well as significant allelic variation in individual KIR genes. As a ing tumor regression. In contrast to HLA-matched transplants, where
consequence, the KIR locus is second only to the HLA locus in the donors and recipients express the same ligands for iKIRs, in HLA-
number of polymorphisms, and unrelated individuals are unlikely to haploidentical SCTs, the recipient may lack the HLA ligand for the
share KIR genotypes. iKIR on a licensed donor NK cell, releasing the cell from inhibition
Distinct HLA class I molecules comprise the ligands for specific and resulting in donor NK cell alloreactivity. Unlicensed donor NK
inhibitory KIRs (iKIRs). An organizing principle of NK cell biology cells may also be activated by a dominance of stimulatory NK cell
is that NK cell self-tolerance is mediated by inhibitory signals deliv- ligands in the inflammatory milieu of a conditioned recipient, but
ered by self HLA class I molecules through iKIRs. The ontogeny of this scenario is not unique to HLA-haploidentical SCT and may also
receptor expression on individual NK cells is poorly understood, but occur after HLA-matched SCT as long as the recipient lacks HLA
it is currently thought that each NK cell expresses at least one inhibi- ligands for iKIR molecules. Although the mechanisms of NK cell
25
tory receptor for a self HLA class I molecule. There are four distinct alloreactivity remain to be fully elucidated, there is great interest in
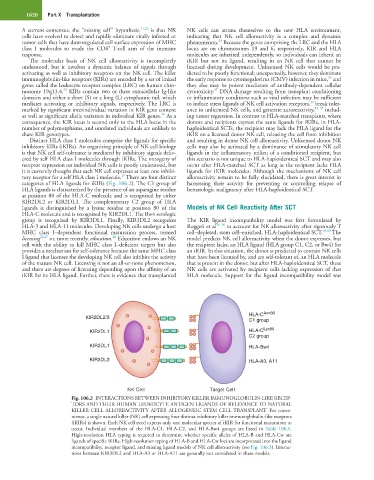
categories of HLA ligands for iKIRs (Fig. 106.2). The C1 group of harnessing their activity for preventing or controlling relapse of
HLA ligands is characterized by the presence of an asparagine residue hematologic malignancy after HLA-haploidentical SCT.
at position 80 of the HLA-C molecule and is recognized by either
KIR2DL2 or KIR2DL3. The complementary C2 group of HLA
ligands is distinguished by a lysine residue at position 80 of the Models of NK Cell Reactivity After SCT
HLA-C molecule and is recognized by KIR2DL1. The Bw4 serologic
group is recognized by KIR3DL1. Finally, KIR3DL2 recognizes The KIR ligand incompatibility model was first formulated by
HLA-3 and HLA-11 molecules. Developing NK cells undergo a host Ruggeri et al 36–38 to account for NK alloreactivity after rigorously T
MHC class I–dependent functional maturation process, termed cell–depleted, stem cell–enriched, HLA-haploidentical SCT. 39,40 The
28
licensing 26,27 or, more recently, education. Education endows an NK model predicts NK cell alloreactivity when the donor expresses, but
cell with the ability to kill MHC class I–deficient targets but also the recipient lacks, an HLA ligand (HLA group C1, C2, or Bw4) for
provides a mechanism for self-tolerance because the same MHC class an iKIR. In this situation, the donor is predicted to contain NK cells
I ligand that licenses the developing NK cell also inhibits the activity that have been licensed by, and are self-tolerant of, an HLA molecule
of the mature NK cell. Licensing is not an all-or-none phenomenon, that is present in the donor, but after HLA-haploidentical SCT, these
and there are degrees of licensing depending upon the affinity of an NK cells are activated by recipient cells lacking expression of that
iKIR for its HLA ligand. Further, there is evidence that transplanted HLA molecule. Support for the ligand incompatibility model was
HLA-C Asn80
KIR2DL2/3
C1 group
KIR2DL1 HLA-C Lys80
C2 group
KIR3DL1 HLA-Bw4
KIR3DL3 HLA-A3, A11
NK Cell Target Cell
Fig. 106.2 INTERACTIONS BETWEEN INHIBITORY KILLER IMMUNOGLOBULIN-LIKE RECEP-
TORS AND THEIR HUMAN LEUKOCYTE ANTIGEN LIGANDS OF RELEVANCE TO NATURAL
KILLER CELL ALLOREACTIVITY AFTER ALLOGENEIC STEM CELL TRANSPLANT. For conve-
nience, a single natural killer (NK) cell expressing four distinct inhibitory killer immunoglobulin-like receptors
(iKIRs) is shown. Each NK cell need express only one molecular species of iKIR for functional maturation to
occur. Individual members of the HLA-C1, HLA-C2, and HLA-Bw4 groups are listed in Table 106.1.
High-resolution HLA typing is required to determine whether specific alleles of HLA-B and HLA-Cw are
ligands of specific iKIRs. High-resolution typing of HLA-B and HLA-Cw loci are incorporated into the ligand
incompatibility, receptor ligand, and missing ligand models of NK cell alloreactivity (see Fig. 106.3). Interac-
tions between KIR3DL2 and HLA-A3 or HLA-A11 are generally not considered in these models.