Page 1926 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1926
1706 Part XI Transfusion Medicine
by some authorities because of the risk of renal damage and renal TABLE Transfusion After ABO Incompatible Hematopoietic
stones due to adenine metabolites. If CPD RBCs are not available, 111.2 Stem Cell Transplantation
the additive solution can be removed and the cells washed. RBCs
preserved in additive solutions also have a lower hematocrit level ABO Group Product Selection
that must be taken into account in making calculations for exchange Donor Recipient Type of Mismatch RBC Plasma/Platelets
transfusion.
The humoral and cellular immune systems of the neonate are A O Major O A
immature, especially in the premature infant. There is a small but A B Bi-directional O AB
real risk of transfusion-induced GVHD in premature infants receiv- A AB Minor A AB
ing RBC transfusions and in the fetus undergoing intrauterine
transfusion. Irradiation of RBCs should be performed in both set- B O Major O B
tings. Another risk of transfusion in low birth weight (<1500 g) B A Bi-directional O AB
premature infants is the development of clinical CMV infection in B AB Minor B AB
infants of CMV-seronegative mothers. CMV safe blood, either CMV
seronegative or leukoreduced, should be provided to these infants. O A Minor O A
Some retrospective studies have suggested that other rare RBC O B Minor O B
transfusion-associated complications in this particularly vulnerable O AB Minor O AB
patient group include necrotizing enterocolitis and intraventricular AB A Major A AB
hemorrhage. However, a causal relationship has yet to be established
through clinical studies. AB B Major B AB
Novel ideas to prevent anemia and decrease donor exposure in AB O Major O AB
premature infants and other neonates include delayed cord clamping, RBC, Red blood cell.
cord milking, umbilical vein blood sampling from the delivered
placenta, and autologous cord blood transfusion. One review of 10
delayed cord clamping studies, and demonstrated lower transfusion
requirements in the delayed versus early clamped group. However, a red cells. Typically, passenger lymphocyte syndrome involves ABO
randomized controlled trial by Strauss et al found no difference in incompatibility, but hemolysis resulting from serologic incompatibil-
transfusion needs between delayed and early clamped groups. A few ity in other blood group systems has been reported. If hemolysis
studies have looked at autologous cord blood transfusions in neonates. increases a few days after transplantation, passenger lymphocyte
One study found that the amount of blood harvested was insufficient syndrome should be considered. Once the donor is engrafted and
to cover all transfusions in low birth weight infants. In addition, incompatible recipient red cells are removed, donor red cells will have
studies have demonstrated that blood processing problems, bacterial normal survival in the recipient. Major incompatibility is defined by
contamination, and costs are all barriers to the routine collection and the presence of blood group antibodies in recipient plasma (e.g.,
autotransfusion of cord blood. group A donor to a group O recipient). Major ABO incompatible
transplantation may result in pure red cell aplasia (PRCA) due to
Red Blood Cell Transfusion in the Allogeneic persisting incompatible ABO antibody targeting the donor’s engraft-
ing erythropoietic precursors expressing ABO antigens. Although
Hematopoietic Stem Cell Transplantation Recipient there may be no evident hemolysis, the donor red cell engraftment
could be further delayed and result in prolonged RBC transfusion
Red cell engraftment is usually the last phase of hematopoietic support. Finally, bidirectional incompatibility is defined as the pres-
recovery after stem cell transplantation; therefore, RBC transfusion ence of incompatible ABO antigens and antibodies contributed by
is common during the posttransplantation period. As hematopoietic both donor and recipient (e.g., group A donor to group B recipient).
stem cells and progenitor cells lack ABO antigens, the transplantation Bidirectional incompatible transplants may cause the problems
outcome is not significantly affected by the red cell antigen/antibody associated with both minor and major ABO incompatible transplants.
incompatibility between the donor and the recipient. However RBC To predict the severity of these complications and provide manage-
transfusion requirements and blood product selection can vary sig- ment, an ABO antibody titer can be performed on either the stem
nificantly depending on the type of ABO incompatibility. The phe- cell product or the recipient. However, titers do not correlate perfectly
notyping of non-ABO/Rh red cell antigens from the donor and with the clinical outcomes. When a high titer incompatible ABO
recipient is not required in the absence of a positive red cell antibody antibody is discovered in a minor incompatibility, the stem cell
screen in the recipient. Patients with an autologous stem cell trans- product can be plasma-reduced to avoid an immediate hemolytic
plant have less RBC transfusion requirements when compared with reaction. Occasionally, in major incompatibilities, plasma exchange
patients receiving allogeneic stem cell transplantation. can be considered if the recipient has a high level of incompatible
In the setting of allogeneic stem cell transplantation, there are four ABO antibody to prevent hemolysis at the time of transplant or
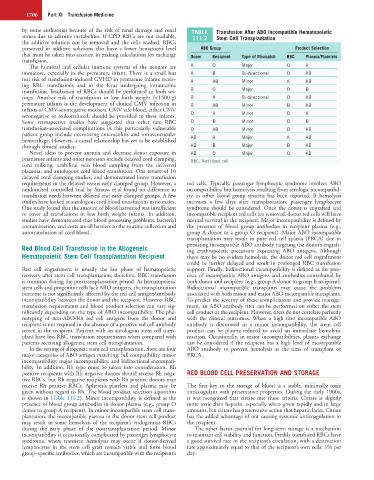
major categories of ABO antigen matching: full compatibility, minor PRCA.
incompatibility, major incompatibility, and bidirectional incompati-
bility. In addition, Rh type must be taken into consideration. Rh
positive recipients with Rh negative donors should receive Rh nega- RED BLOOD CELL PRESERVATION AND STORAGE
tive RBCs, but Rh negative recipients with Rh positive donors may
receive Rh positive RBCs. Apheresis platelets and plasma may be The first key to the storage of blood is a stable, minimally toxic
given without regard to Rh. The blood product selection algorithm anticoagulant with preservative properties. During the early 1900s,
is shown in (Table 111.2). Minor incompatibility is defined as the it was recognized that citrate met these criteria. Citrate is slightly
presence of blood group antibodies in donor plasma (e.g., group O more toxic than heparin, especially when given rapidly and in large
donor to group A recipient). In minor incompatible stem cell trans- amounts, but citrate has preservative action that heparin lacks. Citrate
plantation, the incompatible plasma in the donor stem cell product has the added advantage of not causing systemic anticoagulation in
may result in some hemolysis of the recipient’s endogenous RBCs the recipient.
during the early phase of the posttransplantation period. Minor The other factor essential for long-term storage is a mechanism
incompatibility is occasionally complicated by passenger lymphocyte to maintain cell viability and function. Freshly transfused RBCs have
syndrome, where transient hemolysis may occur if donor-derived a good survival rate in the recipient’s circulation, with a destruction
lymphocytes in the stem cell graft remain viable and form blood rate approximately equal to that of the recipient’s own cells: 1% per
group–specific antibodies, which are incompatible with the recipient’s day.