Page 197 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 197
Chapter 14 Interactions Between Hematopoietic Stem and Progenitor Cells and the Bone Marrow 149
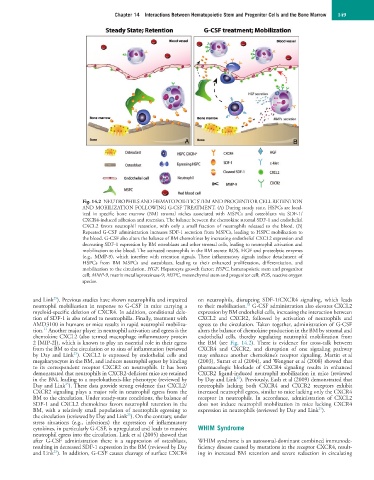
Fig. 14.2 NEUTROPHILS AND HEMATOPOIETIC STEM AND PROGENITOR CELL RETENTION
AND MOBILIZATION FOLLOWING G-CSF TREATMENT. (A) During steady state, HSPCs are local-
ized in specific bone marrow (BM) stromal niches associated with MSPCs and osteoblasts via SDF-1/
CXCR4-induced adhesion and retention. The balance between the chemokine stromal SDF-1 and endothelial
CXCL2 favors neutrophil retention, with only a small fraction of neutrophils released to the blood. (B)
Repeated G-CSF administration increases SDF-1 secretion from MSPCs, leading to HSPC mobilization to
the blood. G-CSF also alters the balance of BM chemokines by increasing endothelial CXCL2 expression and
decreasing SDF-1 expression by BM osteoblasts and other stromal cells, leading to neutrophil activation and
mobilization to the blood. The activated neutrophils in the BM secrete ROS, HGF and proteolytic enzymes
(e.g., MMP-9), which interfere with retention signals. These inflammatory signals induce detachment of
HSPCs from BM MSPCs and osteoblasts, leading to their enhanced proliferation, differentiation, and
mobilization to the circulation. HGF, Hepatocyte growth factor; HSPC, hematopoietic stem and progenitor
cell; MMP-9, matrix metalloproteinase-9; MSPC, mesenchymal stem and progenitor cell; ROS, reactive oxygen
species.
25
and Link ). Previous studies have shown neutrophilia and impaired on neutrophils, disrupting SDF-1/CXCR4 signaling, which leads
28
neutrophil mobilization in response to G-CSF in mice carrying a to their mobilization. G-CSF administration also elevates CXCL2
myeloid-specific deletion of CXCR4. In addition, conditional dele- expression by BM endothelial cells, increasing the interaction between
tion of SDF-1 is also related to neutrophilia. Finally, treatment with CXCL2 and CXCR2, followed by activation of neutrophils and
AMD3100 in humans or mice results in rapid neutrophil mobiliza- egress to the circulation. Taken together, administration of G-CSF
11
tion. Another major player in neutrophil activation and egress is the alters the balance of chemokine production in the BM by stromal and
chemokine CXCL2 (also termed macrophage inflammatory protein endothelial cells, thereby regulating neutrophil mobilization from
2 [MIP-2]), which is known to play an essential role in their egress the BM (see Fig. 14.2). There is evidence for cross-talk between
from the BM to the circulation or to sites of inflammation (reviewed CXCR4 and CXCR2, and disruption of one signaling pathway
25
by Day and Link ). CXCL2 is expressed by endothelial cells and may enhance another chemokine’s receptor signaling. Martin et al
megakaryocytes in the BM, and induces neutrophil egress by binding (2003), Suratt et al (2004), and Wengner et al (2008) showed that
to its correspondent receptor CXCR2 on neutrophils. It has been pharmacologic blockade of CXCR4 signaling results in enhanced
demonstrated that neutrophils in CXCR2-deficient mice are retained CXCR2 ligand-induced neutrophil mobilization in mice (reviewed
25
in the BM, leading to a myelokathexis-like phenotype (reviewed by by Day and Link ). Previously, Eash et al (2009) demonstrated that
25
Day and Link ). These data provide strong evidence that CXCL2/ neutrophils lacking both CXCR4 and CXCR2 receptors exhibit
CXCR2 signaling plays a major role in neutrophil egress from the increased neutrophil egress, similar to mice lacking only the CXCR4
BM to the circulation. Under steady-state conditions, the balance of receptor in neutrophils. In accordance, administration of CXCL2
SDF-1 and CXCL2 chemokines favors neutrophil retention in the does not induce neutrophil mobilization in mice lacking CXCR4
25
BM, with a relatively small population of neutrophils egressing to expression in neutrophils (reviewed by Day and Link ).
25
the circulation (reviewed by Day and Link ). On the contrary, under
stress situations (e.g., infections) the expression of inflammatory
cytokines, in particularly G-CSF, is upregulated and leads to massive WHIM Syndrome
neutrophil egress into the circulation. Link et al (2005) showed that
after G-CSF administration there is a suppression of osteoblasts, WHIM syndrome is an autosomal-dominant combined immunode-
resulting in decreased SDF-1 expression in the BM (reviewed by Day ficiency disease caused by mutations in the receptor CXCR4, result-
25
and Link ). In addition, G-CSF causes cleavage of surface CXCR4 ing in increased BM retention and severe reduction in circulating