Page 2056 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2056
Chapter 121 Pediatric Transfusion Medicine 1825
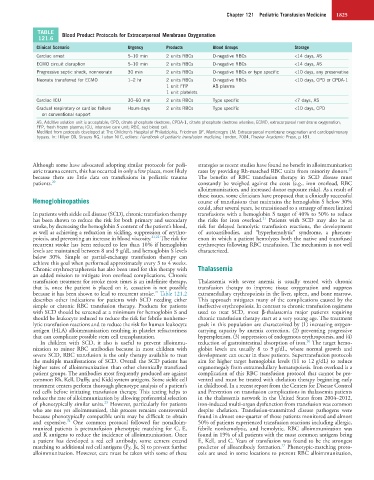
TABLE Blood Product Protocols for Extracorporeal Membrane Oxygenation
121.6
Clinical Scenario Urgency Products Blood Groups Storage
Cardiac arrest 5–10 min 2 units RBCs O-negative RBCs <14 days, AS
ECMO circuit disruption 5–10 min 2 units RBCs O-negative RBCs <14 days, AS
Progressive septic shock, nonneonate 30 min 2 units RBCs O-negative RBCs or type specific <10 days, any preservative
Neonate transferred for ECMO 1–2 hr 2 units RBCs O-negative RBCs <10 days, CPD or CPDA-1
1 unit FFP AB plasma
1 unit platelets
Cardiac ICU 30–60 min 2 units RBCs Type specific <7 days, AS
Gradual respiratory or cardiac failure Hours-days 2 units RBCs Type specific <10 days, CPD
on conventional support
AS, Additive solution unit is acceptable; CPD, citrate phosphate dextrose; CPDA-1, citrate phosphate dextrose adenine; ECMO, extracorporeal membrane oxygenation;
FFP, fresh frozen plasma; ICU, intensive care unit; RBC, red blood cell.
Modified from protocols developed at The Children’s Hospital of Philadelphia. Friedman DF, Montenegro LM: Extracorporeal membrane oxygenation and cardiopulmonary
bypass. In: Hillyer CD, Strauss RG, Luban NLC, editors: Handbook of pediatric transfusion medicine, London, 2004, Elsevier Academic Press, p 181.
Although some have advocated adopting similar protocols for pedi- strategies as recent studies have found no benefit in alloimmunization
25
atric trauma centers, this has occurred in only a few places, most likely rates by providing Rh-matched RBC units from minority donors.
because there are little data on transfusions in pediatric trauma The benefits of RBC transfusion therapy in SCD disease must
patients. 20 constantly be weighed against the costs (e.g., iron overload, RBC
alloimmunization, and increased donor exposure risks). As a result of
these issues, some clinicians have proposed that a clinically successful
Hemoglobinopathies course of transfusions that maintains the hemoglobin S below 30%
could, after several years, be transitioned to a strategy of more limited
In patients with sickle cell disease (SCD), chronic transfusion therapy transfusions with a hemoglobin S target of 40% to 50% to reduce
21
has been shown to reduce the risk for both primary and secondary the risks for iron overload. Patients with SCD may also be at
stroke, by decreasing the hemoglobin S content of the patient’s blood, risk for delayed hemolytic transfusion reactions, the development
as well as achieving a reduction in sickling, suppression of erythro- of autoantibodies, and “hyperhemolytic” syndrome, a phenom-
poiesis, and preventing an increase in blood viscosity. 21,22 The risk for enon in which a patient hemolyzes both the native and transfused
recurrent stroke has been reduced to less than 10% if hemoglobin erythrocytes following RBC transfusion. The mechanism is not well
levels are maintained between 8 and 9 g/dL and hemoglobin S levels characterized.
below 30%. Simple or partial-exchange transfusion therapy can
achieve this goal when performed approximately every 3 to 4 weeks.
Chronic erythrocytapheresis has also been used for this therapy with Thalassemia
an added mission to mitigate iron overload complications. Chronic
transfusion treatment for stroke most times is an indefinite therapy, Thalassemia with severe anemia is usually treated with chronic
that is, once the patient is placed on it, cessation is not possible transfusion therapy to improve tissue oxygenation and suppress
21
because it has been shown to lead to recurrent stroke. Table 121.2 extramedullary erythropoiesis in the liver, spleen, and bone marrow.
describes other indications for patients with SCD needing either This approach mitigates many of the complications caused by the
simple or chronic RBC transfusion therapy. Products for patients ineffective erythropoiesis. In contrast to chronic transfusion regimens
with SCD should be screened at a minimum for hemoglobin S and used to treat SCD, most β-thalassemia major patients requiring
should be leukocyte reduced to reduce the risk for febrile nonhemo- chronic transfusion therapy start at a very young age. The treatment
lytic transfusion reactions and to reduce the risk for human leukocyte goals in this population are characterized by (1) increasing oxygen-
antigen (HLA) alloimmunization resulting in platelet refractoriness carrying capacity by anemia correction, (2) preventing progressive
that can complicate possible stem cell transplantation. hypersplenism, (3) suppression of endogenous erythropoiesis, and (4)
26
In children with SCD, it also is useful to prevent alloimmu- reduction of gastrointestinal absorption of iron. The target hemo-
nization to minor RBC antibodies because in most children with globin levels are usually 8 to 9 g/dL, where normal growth and
severe SCD, RBC transfusion is the only therapy available to treat development can occur in these patients. Supertransfusion protocols
the multiple manifestations of SCD. Overall the SCD patient has aim for higher target hemoglobin levels (11 to 12 g/dL) to reduce
higher rates of alloimmunization than other chronically transfused organomegaly from extramedullary hematopoiesis. Iron overload is a
patient groups. The antibodies most frequently produced are against complication of this RBC transfusion protocol that cannot be pre-
common Rh, Kell, Duffy, and Kidd system antigens. Some sickle cell vented and must be treated with chelation therapy beginning early
treatment centers perform thorough phenotype analysis of a patient’s in childhood. In a recent report from the Centers for Disease Control
red cells before initiating transfusion therapy. This testing helps to and Prevention on transfusion complications in thalassemia patients
reduce the rate of alloimmunization by allowing preferential selection in the thalassemia network in the United States from 2004–2012,
23
of phenotypically similar units. However, particularly for patients iron-induced multi-organ dysfunction from transfusion was common
who are not yet alloimmunized, this process remains controversial despite chelation. Transfusion-transmitted disease pathogens were
because phenotypically compatible units may be difficult to obtain found in almost one-quarter of those patients monitored and almost
24
and expensive. One common protocol followed for nonalloim- 50% of patients experienced transfusion reactions including allergic,
munized patients is pretransfusion phenotypic matching for C, E, febrile nonhemolytic, and hemolytic. RBC alloimmunization was
and K antigens to reduce the incidence of alloimmunization. Once found in 19% of all patients with the most common antigens being
a patient has developed a red cell antibody, some centers extend E, Kell, and C. Years of transfusion was found to be the strongest
27
matching to additional red cell antigens (Fy, Jk, S) to prevent further predictor of alloantibody formation. Phenotypic-matching proto-
alloimmunization. However, care must be taken with some of these cols are used in some locations to prevent RBC alloimmunization,