Page 2157 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2157
1910 Part XII Hemostasis and Thrombosis
A2
N D’ D3 A1 A3 D4 B1-3 C1 C2 CK C
FLOW
Gplb site A2 Tyr1605 collagen
Met1606
N D’ D3 A1 A3 D4 B1-3 C1 C2 CK
A
K2 K2
K3
K1 K1
GPI
N C basic region N
B Alpha Isoform Beta Isoform
TM
1
EGFs
2
llai
3
lla serpin 4 PC
5
lla
6
CS
GAGs EPCR
C D C-terminus
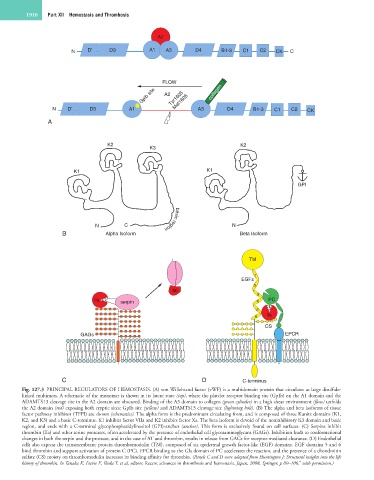
Fig. 127.3 PRINCIPAL REGULATORS OF HEMOSTASIS. (A) von Willebrand factor (vWF) is a multidomain protein that circulates as large disulfide-
linked multimers. A schematic of the monomer is shown in its latent state (top), where the platelet receptor binding site (GpIb) on the A1 domain and the
ADAMTS13 cleavage site in the A2 domain are obscured. Binding of the A3 domain to collagen (green cylinder) in a high shear environment (flow) unfolds
the A2 domain (red) exposing both cryptic sites: GpIb site (yellow) and ADAMTS13 cleavage site (lightning bolt). (B) The alpha and beta isoforms of tissue
factor pathway inhibitor (TFPI) are shown (schematics). The alpha form is the predominant circulating form, and is composed of three Kunitz domains (K1,
K2, and K3) and a basic C-terminus. K1 inhibits factor VIIa and K2 inhibits factor Xa. The beta isoform is devoid of the noninhibitory K3 domain and basic
region, and ends with a C-terminal glycophosphatidylinositol (GPI)-anchor (anchor). This form is exclusively found on cell surfaces. (C) Serpins inhibit
thrombin (IIa) and other serine proteases, often accelerated by the presence of endothelial cell glycosaminoglycans (GAGs). Inhibition leads to conformational
changes in both the serpin and the protease, and in the case of AT and thrombin, results in release from GAGs for receptor-mediated clearance. (D) Endothelial
cells also express the transmembrane protein thrombomodulin (TM), composed of six epidermal growth factor-like (EGF) domains. EGF domains 5 and 6
bind thrombin and support activation of protein C (PC). EPCR binding to the Gla domain of PC accelerates the reaction, and the presence of a chondroitin
sulfate (CS) moiety on thrombomodulin increases its binding affinity for thrombin. (Panels C and D were adapted from Huntington J: Structural insights into the life
4
history of thrombin. In Tanaka K, Davie E, Ikeda Y, et al, editors: Recent advances in thrombosis and hemostasis, Japan, 2008, Springer, p 80–106, with permission.)