Page 2280 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2280
Chapter 136 Inhibitors in Hemophilias 2027
78
binds to platelet membrane-associated phosphatidylserine. Neutral- 100
izing alloantibody inhibitors can interrupt this complex process (see
Fig. 136.1) by (a) preventing FVIII interaction with vWF, thereby 75
significantly decreasing its circulating half-life, (b) hampering the
release of FVIIIa from vWF following thrombin activation, (c)
increasing the time available for FVIII inactivation, and/or (d) pre- 50
venting C2 domains from binding to the phospholipid layer. 79–83
Other FVIII alloantibody inhibitors interact with the A2 domain,
predominantly at the Arg4844–Ile5085 epitope and interrupt Corrected percent residual factor VIII
FVIIIa–FIXa interactions, thereby impairing intrinsic tenase activ- 25
84
ity. Regions outside the A2 and C2 domains are minor epitopes for
FVIII inhibitors; however, those binding to the A3 domain also
84
blocks the FVIIIa–FIXa interaction. In most hemophilia A patients,
alloantibodies recognize multiple epitopes in both the A2 and C2
domains, in contrast to autoantibodies to FVIII, which target either 10
84
the C2 or A2 domain but not both. Although antibodies that target 0 0.5 1 1.5 2
B domain epitopes have been reported, these do not exert any neu-
tralizing activity. Bethesda units per mL plasma
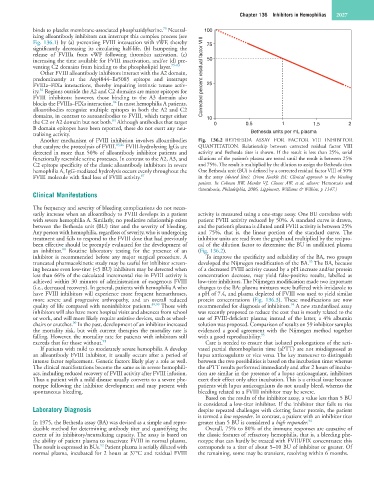
Another mechanism of FVIII inhibition involves alloantibodies Fig. 136.2 BETHESDA ASSAY FOR FACTOR VIII INHIBITOR
that catalyze the proteolysis of FVIII. 85,86 FVIII-hydrolyzing IgGs are QUANTITATION. Relationship between corrected residual factor VIII
detected in more than 50% of alloantibody inhibitor patients and activity and Bethesda titer is shown. If the result is less than 25%, serial
functionally resemble serine proteases. In contrast to the A2, A3, and dilutions of the patient’s plasma are tested until the result is between 25%
C2 epitope specificity of the classic alloantibody inhibitors in severe and 75%. The result is multiplied by the dilution to assign the Bethesda titer.
hemophilia A, IgG-mediated hydrolysis occurs evenly throughout the One Bethesda unit (BU) is defined by a corrected residual factor VIII of 50%
FVIII molecule with final loss of FVIII activity. 87 in the assay (dotted line). (From Konkle BA: Clinical approach to the bleeding
patient. In Colman RW, Marder VJ, Clowes AW, et al, editors: Hemostasis and
thrombosis, Philadelphia, 2006, Lippincott, Williams & Wilkins, p 1147.)
Clinical Manifestations
The frequency and severity of bleeding complications do not neces-
sarily increase when an alloantibody to FVIII develops in a patient activity is measured using a one-stage assay. One BU correlates with
with severe hemophilia A. Similarly, no predictive relationship exists patient FVIII activity reduced by 50%. A standard curve is drawn,
between the Bethesda unit (BU) titer and the severity of bleeding. and the patient’s plasma is diluted until FVIII activity is between 25%
Any person with hemophilia, regardless of severity, who is undergoing and 75%, that is, the linear portion of the standard curve. The
treatment and fails to respond to the FVIII dose that had previously inhibitor units are read from the graph and multiplied by the recipro-
been effective should be promptly evaluated for the development of cal of the dilution factor to determine the BU in undiluted plasma
88
an inhibitor. Routine laboratory testing for the presence of an (Fig. 136.2).
inhibitor is recommended before any major surgical procedure. A To improve the specificity and reliability of the BA, two groups
93
truncated pharmacokinetic study may be useful for inhibitor screen- developed the Nijmegen modification of the BA. The BA, because
ing because even low-titer (<5 BU) inhibitors may be detected when of a decreased FVIII activity caused by a pH increase and/or protein
less than 66% of the calculated incremental rise in FVIII activity is concentration decrease, may yield false-positive results, labelled as
achieved within 30 minutes of administration of exogenous FVIII low-titer inhibitors. The Nijmegen modification made two important
(i.e., decreased recovery). In general, patients with hemophilia A who changes to the BA: plasma mixtures were buffered with imidazole to
have FVIII inhibitors will experience more frequent hemarthroses, a pH of 7.4, and plasma depleted of FVIII was used to yield similar
more severe and progressive arthropathy, and an overall reduced protein concentrations (Fig. 136.3). These modifications are now
94
quality of life compared with noninhibitor patients. 89,90 Those with recommended for diagnosis of inhibitors. A new standardized assay
inhibitors will also have more hospital visits and absences from school was recently proposed to reduce the cost that is mostly related to the
or work, and will more likely require assistive devices, such as wheel- use of FVIII-deficient plasma; instead of the latter, a 4% albumin
90
chairs or crutches. In the past, development of an inhibitor increased solution was proposed. Comparison of results on 59 inhibitor samples
the mortality risk, but with current therapies the mortality rate is evidenced a good agreement with the Nijmegen method together
falling. However, the mortality rate for patients with inhibitors still with a good reproducibility. 95
exceeds that for those without. 91 Care is needed to ensure that isolated prolongations of the acti-
If patients with mild to moderately severe hemophilia A develop vated partial thromboplastin time (aPTT) are not misdiagnosed as
an alloantibody FVIII inhibitor, it usually occurs after a period of lupus anticoagulants or vice versa. The key maneuver to distinguish
intense factor replacement. Genetic factors likely play a role as well. between the two possibilities is based on the incubation time: whereas
The clinical manifestations become the same as in severe hemophili- the aPTT results performed immediately and after 2 hours of incuba-
acs, including reduced recovery of FVIII activity after FVIII infusion. tion are similar in the presence of a lupus anticoagulant, inhibitors
Thus a patient with a mild disease usually converts to a severe phe- exert their effect only after incubation. This is a critical issue because
notype following the inhibitor development and may present with patients with lupus anticoagulants do not usually bleed, whereas the
spontaneous bleeding. bleeding related to a FVIII inhibitor may be severe.
Based on the results of the inhibitor assay, a value less than 5 BU
is considered a low-titer inhibitor. If the inhibitor titer fails to rise
Laboratory Diagnosis despite repeated challenges with clotting factor protein, the patient
is termed a low responder. In contrast, a patient with an inhibitor titer
In 1975, the Bethesda assay (BA) was devised as a simple and repro- greater than 5 BU is considered a high responder. 94
ducible method for determining antibody titer and quantifying the Overall, 75% to 80% of the immune responses are causative of
extent of its inhibitory/neutralizing capacity. The assay is based on the classic features of refractory hemophilia, that is, a bleeding phe-
the ability of patient plasma to inactivate FVIII in normal plasma. notype that can hardly be treated with FVIII/FIX concentrates: this
92
The result is expressed in BUs. Patient plasma is serially diluted with corresponds to a titer of about 5–10 BU of inhibitor or greater. Of
normal plasma, incubated for 2 hours at 37°C and residual FVIII the remaining, some may be transient, resolving within 6 months.