Page 2337 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2337
Chapter 140 Hypercoagulable States 2079
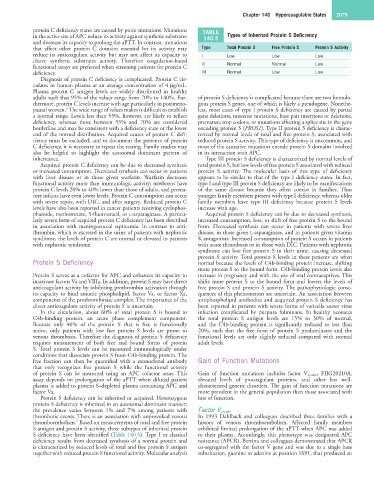
protein C deficiency states are caused by point mutations. Mutations TABLE
in the active site of APC reduce its activity against synthetic substrates 140.5 Types of Inherited Protein S Deficiency
and decrease its capacity to prolong the aPTT. In contrast, mutations
that affect other protein C domains essential for its activity may Type Total Protein S Free Protein S Protein S Activity
reduce its anticoagulant activity but may not affect its capacity to I Low Low Low
cleave synthetic substrates activity. Therefore coagulation-based
functional assays are preferred when screening patients for protein C II Normal Normal Low
deficiency. III Normal Low Low
Diagnosis of protein C deficiency is complicated. Protein C cir-
culates in human plasma at an average concentration of 4 µg/mL.
Plasma protein C antigen levels are widely distributed in healthy
adults such that 95% of the values range from 70% to 140%. Fur- of protein S deficiency is complicated because there are two homolo-
thermore, protein C levels increase with age particularly in postmeno- gous protein S genes, one of which is likely a pseudogene. Nonethe-
6
pausal women. The wide range of values makes it difficult to establish less, most cases of type I protein S deficiency are caused by partial
a normal range. Levels less than 55%, however, are likely to reflect gene deletions, missense mutations, base pair insertions or deletions,
deficiency, whereas those between 55% and 70% are considered premature stop codons, or mutations affecting a splice site in the gene
borderline and may be consistent with a deficiency state or the lower encoding protein S (PROS1). Type II protein S deficiency is charac-
end of the normal distribution. Acquired causes of protein C defi- terized by normal levels of total and free protein S, associated with
ciency must be excluded, and to document the presence of protein reduced protein S activity. This type of deficiency is uncommon, and
C deficiency, it is necessary to repeat the testing. Family studies may most of the causative mutations encode protein S domains involved
also be helpful to highlight the autosomal dominant pattern of in its interaction with APC.
inheritance. Type III protein S deficiency is characterized by normal levels of
Acquired protein C deficiency can be due to decreased synthesis total protein S, but low levels of free protein S associated with reduced
or increased consumption. Decreased synthesis can occur in patients protein S activity. The molecular basis of this type of deficiency
with liver disease or in those given warfarin. Warfarin decreases appears to be similar to that of the type I deficiency states. In fact,
functional activity more than immunologic activity; newborns have type I and type III protein S deficiency are likely to be manifestations
protein C levels 20% to 40% lower than those of adults, and prema- of the same disease because they often coexist in families. Thus
ture infants have even lower levels. Protein C consumption can occur younger family members present with type I deficiency, whereas older
with severe sepsis, with DIC, and after surgery. Reduced protein C family members have type III deficiency because protein S levels
levels have also been reported in cancer patients receiving cyclophos- increase with age.
phamide, methotrexate, 5-fluorouracil, or L-asparaginase. A particu- Acquired protein S deficiency can be due to decreased synthesis,
larly severe form of acquired protein C deficiency has been described increased consumption, loss, or shift of free protein S to the bound
in association with meningococcal septicemia. In contrast to anti- form. Decreased synthesis can occur in patients with severe liver
thrombin, which is excreted in the urine of patients with nephrotic disease, in those given L-asparaginase, and in patients given vitamin
syndrome, the levels of protein C are normal or elevated in patients K antagonists. Increased consumption of protein S occurs in patients
with nephrotic syndrome. with acute thrombosis or in those with DIC. Patients with nephrotic
syndrome can lose free protein S in their urine, causing decreased
protein S activity. Total protein S levels in these patients are often
Protein S Deficiency normal because the levels of C4b-binding protein increase, shifting
more protein S to the bound form. C4b-binding protein levels also
Protein S serves as a cofactor for APC and enhances its capacity to increase in pregnancy and with the use of oral contraceptives. This
inactivate factors Va and VIIIa. In addition, protein S may have direct shifts more protein S to the bound form and lowers the levels of
anticoagulant activity by inhibiting prothrombin activation through free protein S and protein S activity. The pathophysiologic conse-
its capacity to bind anionic phospholipid, factor Va, or factor Xa, quences of this phenomenon are uncertain. An association between
components of the prothrombinase complex. The importance of the antiphospholipid antibodies and acquired protein S deficiency has
direct anticoagulant activity of protein S is uncertain. been reported in patients with severe forms of varicella zoster virus
In the circulation, about 60% of total protein S is bound to infection complicated by purpura fulminans. In healthy neonates
C4b-binding protein, an acute phase complement component. the total protein S antigen levels are 15% to 30% of normal,
Because only 40% of the protein S that is free is functionally and the C4b-binding protein is significantly reduced to less than
active, only patients with low free protein S levels are prone to 20%, such that the free form of protein S predominates and the
venous thrombosis. Therefore the diagnosis of protein S deficiency functional levels are only slightly reduced compared with normal
requires measurement of both free and bound forms of protein adult levels.
S. Total protein S levels can be measured immunologically under
conditions that dissociate protein S from C4b-binding protein. The
free fraction can then be quantified with a monoclonal antibody Gain of Function Mutations
that only recognizes free protein S while the functional activity
of protein S can be measured using an APC cofactor assay. This Gain of function mutations includes factor V Leiden , FIIG20210A,
assay depends on prolongation of the aPTT when diluted patient elevated levels of procoagulant proteins, and other less well-
plasma is added to protein S–depleted plasma containing APC and characterized genetic disorders. The gain of function mutations are
factor Va. more prevalent in the general population than those associated with
Protein S deficiency can be inherited or acquired. Heterozygous loss of function.
protein S deficiency is inherited in an autosomal dominant manner;
the prevalence varies between 1% and 7% among patients with Factor V Leiden
thrombotic events. There is an association with unprovoked venous In 1993 Dahlback and colleagues described three families with a
7
thromboembolism. Based on measurements of total and free protein history of venous thromboembolism. Affected family members
S antigen and protein S activity, three subtypes of inherited protein exhibited limited prolongation of the aPTT when APC was added
S deficiency have been identified (Table 140.5). Type I or classical to their plasma. Accordingly, this phenotype was designated APC
deficiency results from decreased synthesis of a normal protein and resistance (APCR). Bertina and colleagues demonstrated that APCR
is characterized by reduced levels of total and free protein S antigen co-segregated with the factor V gene and was due to a single base
together with reduced protein S functional activity. Molecular analysis substitution, guanine to adenine at position 1691, that produced an