Page 2354 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2354
2096 Part XII Hemostasis and Thrombosis
300 fetal demise, intrauterine growth restriction, and oligohydramnios
Mean +2 SD
280 than aPL antibody–negative pregnant women.
Women with obstetric APS generally present with a history of
260 recurrent (i.e., three or more) miscarriages. In approximately half of
the patients, the pregnancy losses occur in the first trimester; other
240
Annexin A5 anticoagulant ratio (%) 220 211 ± 35 206 ± 37 218 ± 36 patients present with later losses, most in the second trimester, but
n = 16
some even later, including stillbirth. The specific pregnancy compli-
Mean –2 SD
200
cations that define obstetric APS include: the absence of another
explanation for the complications, three or more recurrent spontane-
n = 70
n = 50
A vs. D: P <.0001
180
B vs. D: P <.0001
ous first trimester miscarriages, or one midtrimester loss, stillbirth,
160
A vs. B: P = NS
140
uterine growth restriction, or oligohydramnios. Pregnant women
A vs. C: P = NS
with APS are also more prone to develop DVT during pregnancy or
B vs. C: P = NS
120 C vs. D: P <.01 episode of preeclampsia, preterm labor, placental abruption, intra-
A B C D the puerperium. Rarely, pregnant patients can develop CAPS.
100 As with the thrombotic manifestations, a prior clinical history of
Obstetric Thrombotic Isolated Normal previous pregnancy loss, complications, or thrombosis is a better pre-
APS APS aPL healthy dictor for future pregnancy loss than the degree of laboratory abnor-
antibodies controls mality. Furthermore, recent studies have shown that positivity on more
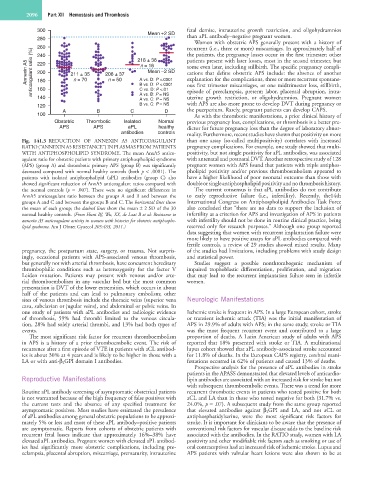
Fig. 141.3 REDUCTION OF ANNEXIN A5 ANTICOAGULANT than one assay (so-called multipositivity) correlates with increased
RATIO (“ANNEXIN A5 RESISTANCE”) IN PLASMAS FROM PATIENTS pregnancy complications. For example, one study showed that multi-
WITH ANTIPHOSPHOLIPID SYNDROME. The mean AnxA5 antico- positivity, but not single positivity for aPL antibodies, was associated
agulant ratio for obstetric patients with primary antiphospholipid syndrome with antenatal and postnatal DVT. Another retrospective study of 128
(APS) (group A) and thrombotic primary APS (group B) was significantly pregnant women with APS found that patients with triple antiphos-
decreased compared with normal healthy controls (both p < .0001). The pholipid positivity and/or previous thromboembolism appeared to
patients with isolated antiphospholipid (aPL) antibodies (group C) also have a higher likelihood of poor neonatal outcome than those with
showed significant reduction of AnxA5 anticoagulant ratios compared with double or single antiphospholipid positivity and no thrombosis history.
the normal controls (p = .007). There were no significant differences in The current consensus is that aPL antibodies do not contribute
AnxA5 anticoagulant ratio between the groups A and B and between the to early reproductive failure (i.e., infertility). Recently, the14th
groups A and C and between the groups B and C. The horizontal lines show International Congress on Antiphospholipid Antibodies Task Force
the mean of each group; the dashed lines show the mean ± 2 SD of the 30 also concluded that “there are no data to support the inclusion of
normal healthy controls. (From Hunt BJ, Wu, XX, de Laat B et al: Resistance to infertility as a criterion for APS and investigation of APS in patients
annexin A5 anticoagulant activity in women with histories for obstetric antiphospho- with infertility should not be done in routine clinical practice, being
lipid syndrome. Am J Obstet Gynecol 205:485, 2011.) reserved only for research purposes.” Although one group reported
data suggesting that women with recurrent implantation failure were
more likely to have positive assays for aPL antibodies compared with
fertile controls, a review of 29 studies showed mixed results. Many
pregnancy, the postpartum state, surgery, or trauma. Not surpris- of the studies had limitations, including problems with study design
ingly, occasional patients with APS-associated venous thrombosis, and statistical power.
but generally not with arterial thrombosis, have concurrent hereditary Studies suggest a possible nonthrombogenic mechanism of
thrombophilic conditions such as heterozygosity for the factor V impaired trophoblastic differentiation, proliferation, and migration
Leiden mutation. Patients may present with venous and/or arte- that may lead to the recurrent implantation failure seen in infertile
rial thromboembolism in any vascular bed but the most common women.
presentation is DVT of the lower extremities, which occurs in about
half of the patients and can lead to pulmonary embolism; other
sites of venous thrombosis include the thoracic veins (superior vena Neurologic Manifestations
cava, subclavian or jugular veins), and abdominal or pelvic veins. In
one study of patients with aPL antibodies and radiologic evidence Ischemic stroke is frequent in APS. In a large European cohort, stroke
of thrombosis, 59% had thrombi limited to the venous circula- or transient ischemic attack (TIA) was the initial manifestation of
tion, 28% had solely arterial thrombi, and 13% had both types of APS in 29.9% of adults with APS; in the same study, stroke or TIA
events. was the most frequent recurrent event and contributed to a large
The most significant risk factor for recurrent thromboembolism proportion of deaths. A Latin American study of adults with APS
in APS is a history of a prior thromboembolic event. The risk of reported that 18% presented with stroke or TIA. A multinational
recurrence after a first episode of VTE in patients with aCL antibod- lupus cohort showed that aPL antibody–associated stroke accounted
ies is about 30% at 4 years and is likely to be higher in those with a for 11.8% of deaths. In the European CAPS registry, cerebral mani-
LA or with anti-β 2 GPI domain I antibodies. festations occurred in 62% of patients and caused 13% of deaths.
Prospective analysis for the presence of aPL antibodies in stroke
patients in the APASS demonstrated that elevated levels of anticardio-
Reproductive Manifestations lipin antibodies are associated with an increased risk for stroke but not
with subsequent thromboembolic events. There was a trend for more
Routine aPL antibody screening of asymptomatic obstetrical patients recurrent thrombotic events in patients who tested positive for both
is not warranted because of the high frequency of false positives with aCL and LA than in those who tested negative for both (31.7% vs.
the current tests and the absence of any specified treatment for 24.0%, p = .07). A subsequent study from the same group reported
asymptomatic positives. Most studies have estimated the prevalence that elevated antibodies against β 2GPI and LA, and not aCL or
of aPL antibodies among general obstetric populations to be approxi- antiphosphatidylserine, were the most significant risk factors for
mately 5% or less and most of these aPL antibody–positive patients stroke. It is important for clinicians to be aware that the presence of
are asymptomatic. Reports from cohorts of obstetric patients with conventional risk factors for vascular disease adds to the baseline risk
recurrent fetal losses indicate that approximately 16%–38% have associated with the antibodies. In the RATIO study, women with LA
elevated aPL antibodies. Pregnant women with elevated aPL antibod- positivity and other modifiable risk factors such as smoking or use of
ies had significantly more obstetric complications, including pre- oral contraceptives had an increased risk of ischemic stroke. Lupus and
eclampsia, placental abruption, miscarriage, prematurity, intrauterine APS patients with valvular heart lesions were also shown to be at