Page 2406 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2406
2148 Part XII Hemostasis and Thrombosis
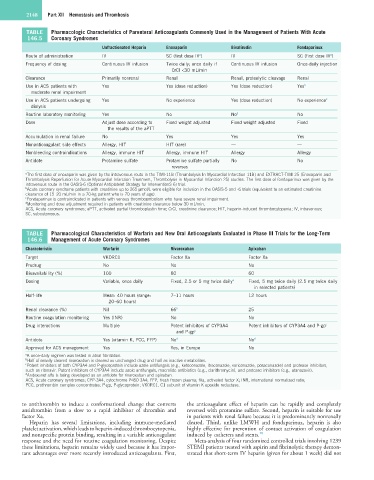
TABLE Pharmacologic Characteristics of Parenteral Anticoagulants Commonly Used in the Management of Patients With Acute
146.5 Coronary Syndromes
Unfractionated Heparin Enoxaparin Bivalirudin Fondaparinux
Route of administration IV SC (first dose IV ) IV SC (first dose IV )
a
a
Frequency of dosing Continuous IV infusion Twice daily; once daily if Continuous IV infusion Once-daily injection
CrCl <30 mL/min
Clearance Primarily nonrenal Renal Renal, proteolytic cleavage Renal
Use in ACS patients with Yes Yes (dose reduction) Yes (dose reduction) Yes b
moderate renal impairment
Use in ACS patients undergoing Yes No experience Yes (dose reduction) No experience c
dialysis
Routine laboratory monitoring Yes No No d No
Dose Adjust dose according to Fixed weight adjusted Fixed weight adjusted Fixed
the results of the aPTT
Accumulation in renal failure No Yes Yes Yes
Nonanticoagulant side effects Allergy, HIT HIT (rare) — —
Nonbleeding contraindications Allergy, immune HIT Allergy, immune HIT Allergy Allergy
Antidote Protamine sulfate Protamine sulfate partially No No
reverses
a The first dose of enoxaparin was given by the intravenous route in the TIMI-11B (Thrombolysis In Myocardial Infarction 11B) and EXTRACT-TIMI 25 (Enoxaparin and
Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment, Thrombolysis in Myocardial Infarction 25) studies. The first dose of fondaparinux was given by the
intravenous route in the OASIS-6 (Optimal Antiplatelet Strategy for InterventionS 6) trial.
b Acute coronary syndrome patients with creatinine up to 265 µmol/L were eligible for inclusion in the OASIS-5 and -6 trials (equivalent to an estimated creatinine
clearance of 15–20 mL/min in a 70-kg patient who is 70 years of age).
c Fondaparinux is contraindicated in patients with venous thromboembolism who have severe renal impairment.
d Monitoring and dose adjustment required in patients with creatinine clearance below 30 mL/min.
ACS, Acute coronary syndromes; aPTT, activated partial thromboplastin time; CrCl, creatinine clearance; HIT, heparin-induced thrombocytopenia; IV, intravenous;
SC, subcutaneous.
TABLE Pharmacological Characteristics of Warfarin and New Oral Anticoagulants Evaluated in Phase III Trials for the Long-Term
146.6 Management of Acute Coronary Syndromes
Characteristic Warfarin Rivaroxaban Apixaban
Target VKORC1 Factor Xa Factor Xa
Prodrug No No No
Bioavailability (%) 100 80 60
Dosing Variable, once daily Fixed, 2.5 or 5 mg twice daily a Fixed, 5 mg twice daily (2.5 mg twice daily
in selected patients)
Half-life Mean: 40 hours (range: 7–11 hours 12 hours
20–60 hours)
Renal clearance (%) Nil 66 b 25
Routine coagulation monitoring Yes (INR) No No
Drug interactions Multiple Potent inhibitors of CYP3A4 Potent inhibitors of CYP3A4 and P-gp c
and P-gp c
Antidote Yes (vitamin K, PCC, FFP) No d No d
Approved for ACS management Yes Yes, in Europe No
a A once-daily regimen was tested in atrial fibrillation.
b Half of renally cleared rivaroxaban is cleared as unchanged drug and half as inactive metabolites.
c Potent inhibitors of both CYP3A4 and P-glycoprotein include azole antifungals (e.g., ketoconazole, itraconazole, voriconazole, posaconazole) and protease inhibitors,
such as ritonavir. Potent inhibitors of CYP3A4 include azole antifungals, macrolide antibiotics (e.g., clarithromycin), and protease inhibitors (e.g., atanazavir).
d Andexanet alfa is being developed as an antidote for rivaroxaban and apixaban.
ACS, Acute coronary syndromes; CYP-3A4, cytochrome P450 3A4; FFP, fresh frozen plasma; fXa, activated factor X; INR, international normalized ratio;
PCC, prothrombin complex concentrates; P-gp, P-glycoprotein; VKORC1, C1 subunit of vitamin K epoxide reductase.
to antithrombin to induce a conformational change that converts the anticoagulant effect of heparin can be rapidly and completely
antithrombin from a slow to a rapid inhibitor of thrombin and reversed with protamine sulfate. Second, heparin is suitable for use
factor Xa. in patients with renal failure because it is predominantly nonrenally
Heparin has several limitations, including immune-mediated cleared. Third, unlike LMWH and fondaparinux, heparin is also
platelet activation, which leads to heparin-induced thrombocytopenia, highly effective for prevention of contact activation of coagulation
and nonspecific protein binding, resulting in a variable anticoagulant induced by catheters and stents. 28
response and the need for routine coagulation monitoring. Despite Meta-analysis of four randomized controlled trials involving 1239
these limitations, heparin remains widely used because it has impor- STEMI patients treated with aspirin and fibrinolytic therapy demon-
tant advantages over more recently introduced anticoagulants. First, strated that short-term IV heparin (given for about 1 week) did not