Page 408 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 408
330 Part IV Disorders of Hematopoietic Cell Development
A B C
D E F G
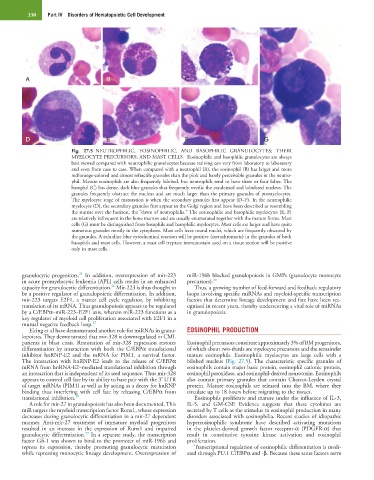
Fig. 27.5 NEUTROPHILIC, EOSINOPHILIC, AND BASOPHILIC GRANULOCYTES; THEIR
MYELOCYTE PRECURSORS; AND MAST CELLS. Eosinophilic and basophilic granulocytes are always
best viewed compared with neutrophilic granulocytes because staining can vary from laboratory to laboratory
and even from case to case. When compared with a neutrophil (A), the eosinophil (B) has larger and more
red/orange-colored and almost refractile granules than the pink and barely perceivable granules in the neutro-
phil. Mature eosinophils are also frequently bilobed, but neutrophils tend to have three or four lobes. The
basophil (C) has dense, dark blue granules that frequently overlie the condensed and lobulated nucleus. The
granules frequently obstruct the nucleus and are much larger than the primary granules of promyelocytes.
The myelocyte stage of maturation is when the secondary granules first appear (D–F). In the neutrophilic
myelocyte (D), the secondary granules first appear in the Golgi region and have been described as resembling
the sunrise over the horizon, the “dawn of neutrophilia.” The eosinophilic and basophilic myelocytes (E, F)
are relatively infrequent in the bone marrow and are usually enumerated together with the mature forms. Mast
cells (G) must be distinguished from basophils and basophilic myelocytes. Mast cells are larger and have quite
numerous granules mostly in the cytoplasm. Mast cells have round nuclei, which are frequently obscured by
the granules. A toluidine blue cytochemical reaction will be positive (metachromatic) in the granules of both
basophils and mast cells. However, a mast cell tryptase immunostain used on a tissue section will be positive
only in mast cells.
25
granulocytic progenitors. In addition, overexpression of mir-223 miR-196b blocked granulopoiesis in GMPs (granulocyte monocyte
in acute promyelocytic leukemia (APL) cells results in an enhanced precursors). 30
26
capacity for granulocytic differentiation. Mir-223 is thus thought to Thus, a growing number of feed-forward and feedback regulatory
be a positive regulator of granulopoietic differentiation. In addition, loops involving specific miRNAs and myeloid-specific transcription
mir-223 targets E2F1, a master cell cycle regulator, by inhibiting factors that determine lineage development and fate have been rec-
translation of its mRNA. Thus granulopoiesis appears to be regulated ognized in recent years, thereby underscoring a vital role of miRNAs
by a C/EBPα–miR-223–E2F1 axis, wherein miR-223 functions as a in granulopoiesis.
key regulator of myeloid cell proliferation associated with E2F1 in a
mutual negative feedback loop. 27
Eiring et al have demonstrated another role for miRNAs in granu- EOSINOPHIL PRODUCTION
lopoiesis. They demonstrated that mir-328 is downregulated in CML
patients in blast crisis. Restoration of mir-328 expression restores Eosinophil precursors constitute approximately 3% of BM progenitors,
differentiation by interaction with both the C/EBPα translational of which about two-thirds are myelocyte precursors and the remainder
inhibitor hnRNP-E2 and the mRNA for PIM1, a survival factor. mature eosinophils. Eosinophilic myelocytes are large cells with a
The interaction with hnRNP-E2 leads to the release of C/EBPα bilobed nucleus (Fig. 27.5). The characteristic specific granules of
mRNA from hnRNA-E2–mediated translational inhibition through eosinophils contain major basic protein, eosinophil cationic protein,
an interaction that is independent of its seed sequence. Thus mir-328 eosinophil peroxidase, and eosinophil-derived neurotoxin. Eosinophils
appears to control cell fate by its ability to base pair with the 3′ UTR also contain primary granules that contain Charcot-Leyden crystal
of target mRNAs (PIM1) as well as by acting as a decoy for hnRNP protein. Mature eosinophils are released into the BM, where they
binding thus interfering with cell fate by releasing C/EBPα from circulate up to 18 hours before migrating to the tissues.
translational inhibition. 28 Eosinophils proliferate and mature under the influence of IL-3,
A role for mir-27 in granulopoiesis has also been documented. This IL-5, and GM-CSF. Evidence suggests that these cytokines are
miR targets the myeloid transcription factor Runx1, whose expression secreted by T cells as the stimulus to eosinophil production in many
decreases during granulocytic differentiation in a mir-27 dependent disorders associated with eosinophilia. Recent studies of idiopathic
manner. Anti-mir-27 treatment of immature myeloid progenitors hypereosinophilic syndrome have described activating mutations
resulted in an increase in the expression of Runx1 and impaired in the platelet-derived growth factor receptor-α (PDGFR-α) that
29
granulocytic differentiation. In a separate study, the transcription result in constitutive tyrosine kinase activation and eosinophil
factor Gfi-1 was shown to bind to the promoter of miR-196b and proliferation.
repress its expression, thereby promoting granulocytic maturation Transcriptional regulation of eosinophilic differentiation is medi-
while repressing monocytic lineage development. Overexpression of ated through PU.1 C/EBPα and -β. Because these same factors serve