Page 467 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 467
388 Part IV Disorders of Hematopoietic Cell Development
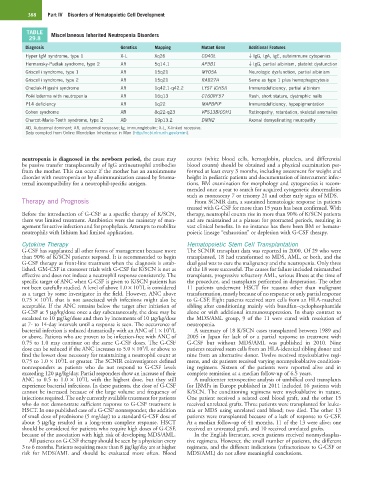
TABLE Miscellaneous Inherited Neutropenia Disorders
29.8
Diagnosis Genetics Mapping Mutant Gene Additional Features
Hyper IgM syndrome, type 1 X-L Xq26 CD4OL ↓ IgG, IgA, IgE, autoimmune cytopenias
Hermansky-Pudlak syndrome, type 2 AR 5q14.1 AP3B1 ↓ IgG, partial albinism, platelet dysfunction
Griscelli syndrome, type 1 AR 15q21 MYO5A Neurologic dysfunction, partial albinism
Griscelli syndrome, type 2 AR 15q21 RAB27A Same as type 1 plus hemophagocytosis
Chediak-Higashi syndrome AR 1q42.1-q42.2 LYST (CHSI) Immunodeficiency, partial albinism
Poikiloderma with neutropenia AR 16q13 C160RF57 Rash, short stature, dystrophic nails
P14 deficiency AR 1q22 MAPBPIP Immunodeficiency, hypopigmentation
Cohen syndrome AR 8q22-q23 VPS13B/COH1 Retinopathy, retardation, skeletal anomalies
Charcot-Marie-Tooth syndrome, type 2 AD 19p13.2 DMN2 Axonal demyelinating neuropathy
AD, Autosomal dominant; AR, autosomal recessive; Ig, immunoglobulin; X-L, X-linked recessive.
Data compiled from Online Mendelian Inheritance in Man (http://ncbi.nlm.nih.gov/omim).
neutropenia is diagnosed in the newborn period, the cause may counts (white blood cells, hemoglobin, platelets, and differential
be passive transfer transplacentally of IgG antineutrophil antibodies blood counts) should be obtained and a physical examination per-
from the mother. This can occur if the mother has an autoimmune formed at least every 3 months, including assessment for weight and
disorder with neutropenia or by alloimmunization caused by fetoma- height in pediatric patients and documentation of intercurrent infec-
ternal incompatibility for a neutrophil-specific antigen. tions. BM examination for morphology and cytogenetics is recom-
mended once a year to search for acquired cytogenetic abnormalities
such as monosomy 7 or trisomy 21 and other early signs of MDS.
Therapy and Prognosis From SCNIR data, a sustained hematologic response in patients
treated with G-CSF for more than 15 years has been confirmed. With
Before the introduction of G-CSF as a specific therapy of K/SCN, therapy, neutrophil counts rise in more than 90% of K/SCN patients
there was limited treatment. Antibiotics were the mainstay of man- and are maintained at a plateau for protracted periods, resulting in
agement for active infection and for prophylaxis. Attempts to mobilize vast clinical benefits. In no instance has there been BM or hemato-
neutrophils with lithium had limited application. poietic lineage “exhaustion” or depletion with G-CSF therapy.
Cytokine Therapy Hematopoietic Stem Cell Transplantation
G-CSF has supplanted all other forms of management because more The SCNIR transplant data was reported in 2000. Of 29 who were
than 90% of K/SCN patients respond. It is recommended to begin transplanted, 18 had transformed to MDS, AML, or both, and the
G-CSF therapy as front-line treatment when the diagnosis is estab- dual goal was to cure the malignancy and the neutropenia. Only three
lished. GM-CSF in crossover trials with G-CSF for K/SCN is not as of the 18 were successful. The causes for failure included mismatched
effective and does not induce a neutrophil response consistently. The transplants, progressive refractory AML, serious illness at the time of
specific target of ANC when G-CSF is given to K/SCN patients has the procedure, and transplants performed in desperation. The other
9
not been carefully studied. A level of above 1.0 × 10 /L is considered 11 patients underwent HSCT for reasons other than malignant
as a target by some investigator in the field. However, ANC above transformation, mostly because of no response or only partial response
9
0.75 × 10 /L that is not associated with infections might also be to G-CSF. Eight patients received stem cells from an HLA-matched
acceptable. If the ANC remains below the target after initiation of sibling after conditioning mainly with busulfan–cyclophosphamide
G-CSF at 5 µg/kg/dose once a day subcutaneously, the dose may be alone or with additional immunosuppression. In sharp contrast to
escalated to 10 µg/kg/dose and then by increments of 10 µg/kg/dose the MDS/AML group, 9 of the 11 were cured with resolution of
at 7- to 14-day intervals until a response is seen. The occurrence of neutropenia.
9
bacterial infection is reduced dramatically with an ANC of 1 × 10 /L A summary of 18 K/SCN cases transplanted between 1989 and
or above. Patients who are proven to be infection-free with ANC of 2005 in Japan for lack of or a partial response to treatment with
0.75 to 1.0 may continue on the same G-CSF doses. The G-CSF G-CSF but without MDS/AML was published in 2010. Nine
9
dose can be reduced if the ANC increases to 5.0 × 10 /L or above to patients received stem cells from an HLA-identical sibling donor and
find the lowest dose necessary for maintaining a neutrophil count at nine from an alternative donor. Twelve received myeloablative regi-
9
0.75 to 1.0 × 10 /L or greater. The SCNIR coinvestigators defined mens, and six patients received varying nonmyeloablative condition-
nonresponders as patients who do not respond to G-CSF levels ing regimens. Sixteen of the patients were reported alive and in
exceeding 120 µg/kg/day. Partial responders show an increase of their complete remission at a median follow-up of 6.5 years.
9
ANC to 0.5 to 1.0 × 10 /L with the highest dose, but they still A multicenter retrospective analysis of umbilical cord transplants
experience bacterial infections. In these patients, the dose of G-CSF for IBMFs in Europe published in 2011 included 16 patients with
cannot be increased because of the large volume and frequency of K/SCN. The conditioning regimens were myeloablative in nature.
injections required. The only currently available treatment for patients One patient received a related cord blood graft, and the other 15
who do not demonstrate sufficient response to G-CSF treatment is received unrelated grafts. Three patients were transplanted for leuke-
HSCT. In one published case of a G-CSF nonresponder, the addition mia or MDS using unrelated cord blood; two died. The other 13
of small dose of prednisone (5 mg/day) to a standard G-CSF dose of patients were transplanted because of a lack of response to G-CSF.
about 5 µg/kg resulted in a long-term complete response. HSCT At a median follow-up of 41 months, 11 of the 13 were alive; one
should be considered for patients who require high doses of G-CSF, received an untreated graft, and 10 received unrelated grafts.
because of the association with high risk of developing MDS/AML. In the English literature, seven patients received nonmyeloaplas-
All patients on G-CSF therapy should be seen by a physician every tive regimens. However, the small number of patients, the different
3 to 6 months. Patients requiring more than 8 µg/kg/day are at higher regimens, and the different indications (refractoriness to G-CSF or
risk for MDS/AML and should be evaluated more often. Blood MDS/AML) do not allow meaningful conclusions.