Page 465 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 465
386 Part IV Disorders of Hematopoietic Cell Development
cells such as CSF1. Patient-related mutations also disable the GFI1 number of reports of short stature, cataracts, microcephaly, seizures,
repressor activity on ELANE expression, which leads to accumulation developmental delay, and mental retardation. As mentioned earlier,
of neutrophil elastase in all subcellular compartments and might neurologic manifestations are part of a small group of patients with
underlie the mechanism for premature apoptosis. Another potential HAX1 mutations. Data from the SCNIR indicate that some patients
mechanism for neutropenia when GFI1 is deficient might be related with K/SCN develop bone demineralization that may be an intrinsic
to loss of GFI1-mediated regulation of the expression of the microR- component of the disorder; it has been observed before and during
NAs miR-21 and miR-196b, which regulate myeloid maturation. G-CSF therapy. The underlying pathogenesis is unclear, but patients
A constitutive point mutation was discovered in the extracellular can develop bone pain and unusual fractures. Osteopenia is a common
domain of the G-CSF receptor (GCSFR or CSF3R) in a patient with theme with the IBMFSs.
K/SCN who also had a mutant ELANE gene. The receptor mutation
affected ligand–receptor complex formation with severe consequences
for intracellular signal transduction; the patient was totally unrespon- Laboratory Findings
sive to G-CSF therapy. As demonstrated in vitro and then clinically,
corticosteroids combined with G-CSF produced a corrective action Peripheral Blood and Bone Marrow
through synergistic activation of STAT5, and the patient responded
to G-CSF therapy. Subsequently additional patients with cell-surface Neutropenia is profound and persistent in K/SCN, usually less than
GCSFR mutations who did not refractory to G-CSF therapy were 200/µL. A small number of patients have intermittent cycling pat-
published, suggesting that this may be a common finding in cases terns with regular periodicity that ranges in a low to severely low
unresponsive to treatment. Importantly, four K/SCN patients from neutrophil count range. A compensatory two- to fourfold increase in
two different families who did not respond to G-CSF treatment were monocytes is seen, sometimes accompanied by eosinophilia. At
found to have biallelic mutations in GCSFR. diagnosis, platelet numbers are normal or increased, and hemoglobin
Regarding the cellular pathology of K/SCN, many cell culture values are usually normal. In survivors in the precytokine era, anemia
studies performed in the 1970s and 1980s provided clonogenic data of chronic disease associated with recurrent infections and inflamma-
that pointed to intrinsically defective granulocytic progenitors. tion was common. Aside from neutropenia, humoral and cellular
Further reports confirmed this and excluded other possible pathoge- immunity is completely normal.
netic factors. To summarize: (1) K/SCN BM myeloid colony growth Bone marrow specimens are usually normocellular. The striking
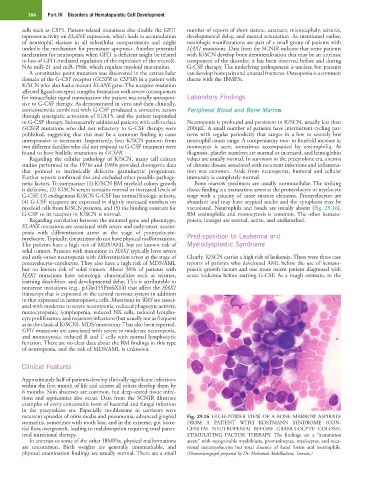
is defective, (2) K/SCN serum contains normal or increased levels of classic finding is a maturation arrest at the promyelocyte or myelocyte
G-CSF, (3) endogenous K/SCN G-CSF has normal biologic activity, stage with a paucity of more mature elements. Promyelocytes are
(4) G-CSF receptors are expressed in slightly increased numbers on abundant and may have atypical nuclei and the cytoplasm may be
myeloid cells from K/SCN patients, and (5) the binding constant for vacuolated. Neutrophils and bands are usually absent (Fig. 29.16).
G-CSF to its receptor in K/SCN is normal. BM eosinophilia and monocytosis is common. The other hemato-
Regarding correlation between the mutated gene and phenotype, poietic lineages are normal, active, and undisturbed.
ELANE mutations are associated with severe and early-onset neutro-
penia with differentiation arrest at the stage of promyelocyte-
myelocyte. Typically, the patients do not have physical malformations. Predisposition to Leukemia and
The patients have a high risk of MDS/AML but no known risk of Myelodysplastic Syndrome
solid tumors. Patients with mutations in HAX1 typically have severe
and early-onset neutropenia with differentiation arrest at the stage of Clearly, K/SCN carries a high risk of leukemia. There were three case
promyelocyte–myelocyte. They also have a high risk of MDS/AML reports of patients who developed AML before the use of hemato-
but no known risk of solid tumors. About 30% of patients with poietic growth factors and one more recent patient diagnosed with
HAX1 mutations have neurologic abnormalities such as seizures, acute leukemia before starting G-CSF. As a rough estimate, in the
learning disabilities, and developmental delay. This is attributable to
nonsense mutations (e.g., p.Gln155ProfsX14) that affect the HAX1
transcript that is expressed in the central nervous system in addition
to that expressed in hematopoietic cells. Mutations in WAS are associ-
ated with moderate to severe neutropenia, reduced phagocyte activity,
monocytopenia, lymphopenia, reduced NK cells, reduced lympho-
cyte proliferation, and recurrent infections (but usually not as frequent
as in the classical K/SCN). MDS/monosomy 7 has also been reported.
GFI1 mutations are associated with severe to moderate neutropenia,
and monocytosis, reduced B and T cells with normal lymphocytic
function. There are no clear data about the BM findings in this type
of neutropenia, and the risk of MDS/AML is unknown.
Clinical Features
Approximately half of patients develop clinically significant infections
within the first month of life and almost all others develop them by
6 months. Skin abscesses are common, but deep-seated tissue infec-
tions and septicemias also occur. Data from the SCNIR illustrate
examples of every conceivable form of bacterial and fungal infection
in the precytokine era. Especially troublesome in survivors were
recurrent episodes of otitis media and pneumonia; advanced gingival Fig. 29.16 HIGH-POWER VIEW OF A BONE MARROW ASPIRATE
stomatitis, sometimes with tooth loss; and in the extreme, gut bacte- FROM A PATIENT WITH KOSTMANN SYNDROME (CON-
rial flora overgrowth, leading to malabsorption requiring total paren- GENITAL NEUTROPENIA) BEFORE GRANULOCYTE COLONY-
teral nutritional therapy. STIMULATING FACTOR THERAPY. The findings are a “maturation
In contrast to some of the other IBMFSs, physical malformations arrest” with recognizable myeloblasts, promyelocytes, myelocytes, and occa-
are uncommon. Birth weights are generally unremarkable, and sional metamyelocytes but total absence of band forms and neutrophils.
physical examination findings are usually normal. There are a small (Photomicrograph prepared by Dr. Mohamed Abdelhaleem, Toronto.)