Page 497 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 497
Chapter 31 Paroxysmal Nocturnal Hemoglobinuria 417
180 Exon 1 2 3 4 5 6
160
120 BP 23 777 133 133 207
2316
Counts 90 Fig. 31.3 STRUCTURE OF THE HUMAN PIGA GENE. Boxes represent
60 exons; intervening lines represent introns. Shaded areas show noncoding
regions.
30
0 Paroxysmal Nocturnal Hemoglobinuria Stem Cell
0 200 400 600 800 1000
A CD59 FITC (normal)
90 PNH is a clonal hematopoietic disorder. The first evidence to support
80 III II I the notion that PNH arises through the mutation of an abnormal
70 multipotent hematopoietic stem cell was derived from glucose
6-phosphate dehydrogenase studies on the red cells of women with
Counts 60 PNH. Subsequently, flow cytometric analyses revealed that all
7
50
hematopoietic lineages—myeloid, erythroid, and lymphoid—were
40
30 involved. Furthermore, PIGA mutations found in granulocytes match
−
+
20 those found in other lineages, and CD34 CD38 stem/progenitor
10 cells have been shown to be missing GPI-anchored proteins in PNH
0 patients. Thus the “hit” in PNH clearly involves a multipotent
0 200 400 600 800 1000 hematopoietic stem cell. In PNH, both B cells and T cells have been
B CD59 FITC (patient) shown to be derived from the malignant clone.
90
80 Paroxysmal Nocturnal Hemoglobinuria Red Cells
70
60
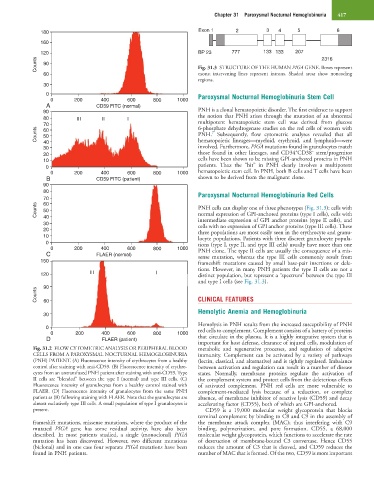
Counts 50 PNH cells can display one of three phenotypes (Fig. 31.3): cells with
normal expression of GPI-anchored proteins (type I cells), cells with
40
30 intermediate expression of GPI anchor proteins (type II cells), and
cells with no expression of GPI anchor proteins (type III cells). These
20 three populations are most easily seen in the erythrocyte and granu-
10 locyte populations. Patients with three discreet granulocyte popula-
0 tions (type I, type II, and type III cells) usually have more than one
0 200 400 600 800 1000 PNH clone. The type II cells are usually the consequence of a mis-
C FLAER (normal) sense mutation, whereas the type III cells commonly result from
150 frameshift mutations caused by small base-pair insertions or dele-
tions. However, in many PNH patients the type II cells are not a
120 III I distinct population, but represent a “spectrum” between the type III
and type I cells (see Fig. 31.3).
90
Counts 60 CLINICAL FEATURES
Hemolytic Anemia and Hemoglobinuria
30
Hemolysis in PNH results from the increased susceptibility of PNH
0 red cells to complement. Complement consists of a battery of proteins
0 200 400 600 800 1000
D FLAER (patient) that circulate in the plasma. It is a highly integrative system that is
important for host defense, clearance of injured cells, modulation of
Fig. 31.2 FLOW CYTOMETRIC ANALYSIS OR PERIPHERAL BLOOD metabolic and regenerative processes, and regulation of adaptive
CELLS FROM A PAROXYSMAL NOCTURNAL HEMOGLOBINURIA immunity. Complement can be activated by a variety of pathways
(PNH) PATIENT. (A) Fluorescence intensity of erythrocytes from a healthy (lectin, classical, and alternative) and is tightly regulated. Imbalance
control after staining with anti-CD59. (B) Fluorescence intensity of erythro- between activation and regulation can result in a number of disease
cytes from an untransfused PNH patient after staining with anti-CD59. Type states. Normally, membrane proteins regulate the activation of
II cells are “blended” between the type I (normal) and type III cells. (C) the complement system and protect cells from the deleterious effects
Fluorescence intensity of granulocytes from a healthy control stained with of activated complement. PNH red cells are more vulnerable to
FLAER. (D) Fluorescence intensity of granulocytes from the same PNH complement-mediated lysis because of a reduction, or complete
patient as (B) following staining with FLAER. Note that the granulocytes are absence, of membrane inhibitor of reactive lysis (CD59) and decay
almost exclusively type III cells. A small population of type I granulocytes is accelerating factor (CD55), both of which are GPI-anchored.
present. CD59 is a 19,000 molecular weight glycoprotein that blocks
terminal complement by binding to C8 and C9 in the assembly of
frameshift mutations, missense mutations, where the product of the the membrane attack complex (MAC); thus interfering with C9
mutated PIGA gene has some residual activity, have also been binding, polymerization, and pore formation. CD55, a 68,000
described. In most patients studied, a single (monoclonal) PIGA molecular weight glycoprotein, which functions to accelerate the rate
mutation has been discovered. However, two different mutations of destruction of membrane-bound C3 convertase. Hence CD55
(biclonal) and in one case four separate PIGA mutations have been reduces the amount of C3 that is cleaved, and CD59 reduces the
found in PNH patients. number of MAC that is formed. Of the two, CD59 is more important