Page 502 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 502
422 Part IV Disorders of Hematopoietic Cell Development
100 of severe thrombocytopenia. Terminal complement blockade appears
Transfusion dependent
90 Transfusion independent to be the most effective way to prevent further thrombotic events
and in many patients may allow for the discontinuation of antico-
80 agulation. Pregnancy, surgery, or the use of oral contraceptives can
Percentage of patients 60 Stem Cell Transplantation
increase the risk for thrombosis in PNH and should be considered
70
high risk.
50
40
30
therapy for PNH, but is associated with significant morbidity and
20 Allogeneic stem cell transplantation (ASCT) is the only curative
25
mortality. The International Bone Marrow Transplant Registry
10 (IBMTR) reported a two-year survival probability of 56% in 48
0 recipients of HLA-identical sibling transplants between 1978 and
Baseline 0 6 6 12 12 18 18 24 24 30 30-36 1995. The median age was 28 years. The majority of the deaths in
Time interval (months) this study occurred within one year of transplantation. One of seven
recipients of alternative donor allogeneic transplants reported to the
Patients(n) 195 195 192 187 179 134 78 IBMTR during this period was alive 5 years after transplant. The
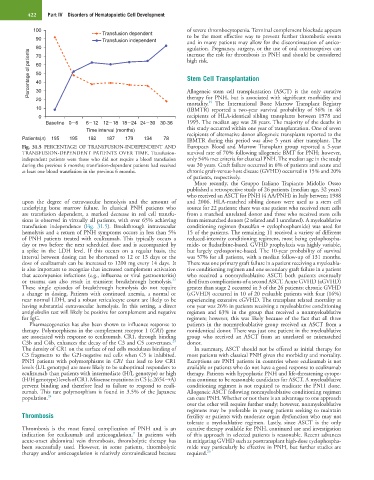
Fig. 31.5 PERCENTAGE OF TRANSFUSION-INDEPENDENT AND European Blood and Marrow Transplant group reported a 5-year
TRANSFUSION-DEPENDENT PATIENTS OVER TIME. Transfusion- survival rate of 70% following allogeneic BMT for PNH; however,
independent patients were those who did not require a blood transfusion only 54% met criteria for classical PNH. The median age in the study
during the previous 6 months; transfusion-dependent patients had received was 30 years. Graft failure occurred in 6% of patients and acute and
at least one blood transfusion in the previous 6 months. chronic graft-versus-host disease (GVHD) occurred in 15% and 20%
of patients, respectively.
More recently, the Gruppo Italiano Trapianto Midollo Osseo
published a retrospective study of 26 patients (median age, 32 years)
who received an ASCT for PNH (4 AA/PNH) in Italy between 1988
upon the degree of extravascular hemolysis and the amount of and 2006. HLA-matched sibling donors were used as a stem cell
underlying bone marrow failure. In classical PNH patients who source for 22 patients; there was one patient who received stem cells
are transfusion dependent, a marked decrease in red cell transfu- from a matched unrelated donor and three who received stem cells
sions is observed in virtually all patients, with over 65% achieving from mismatched donors (2 related and 1 unrelated). A myeloablative
transfusion independence (Fig. 31.5). Breakthrough intravascular conditioning regimen (busulfan + cyclophosphamide) was used for
hemolysis and a return of PNH symptoms occurs in less than 5% 15 of the patients. The remaining 11 received a variety of different
of PNH patients treated with eculizumab. This typically occurs a reduced-intensity conditioning regimens, most being cyclophospha-
day or two before the next scheduled dose and is accompanied by mide- or fludaribine-based. GVHD prophylaxis was highly variable,
a spike in the LDH level. If this occurs on a regular basis, the but largely cyclosporine-based. The 10-year probability of survival
interval between dosing can be shortened to 12 or 13 days or the was 57% for all patients, with a median follow-up of 131 months.
dose of eculizumab can be increased to 1200 mg every 14 days. It There was one primary graft failure in a patient receiving a myeloabla-
is also important to recognize that increased complement activation tive conditioning regimen and one secondary graft failure in a patient
that accompanies infections (e.g., influenza or viral gastroenteritis) who received a nonmyeloablative ASCT; both patients eventually
22
or trauma can also result in transient breakthrough hemolysis. died from complications of a second ASCT. Acute GVHD (aGVHD)
These single episodes of breakthrough hemolysis do not require greater than stage 2 occurred in 3 of the 26 patients; chronic GVHD
a change in dosing. Patients with continued anemia, a normal or (cGVHD) occurred in 10 of 20 evaluable patients with four (16%)
near normal LDH, and a robust reticulocyte count are likely to be experiencing extensive cGVHD. The transplant related mortality at
having substantial extravascular hemolysis. In this setting, a direct one year was 26% in patients receiving a myeloablative conditioning
antiglobulin test will likely be positive for complement and negative regimen and 63% in the group that received a nonmyeloablative
for IgG. regimen; however, this was likely because of the fact that all three
Pharmacogenetics has also been shown to influence response to patients in the nonmyeloablative group received an ASCT from a
therapy. Polymorphisms in the complement receptor 1 (CR1) gene nonidentical donor. There was just one patient in the myeloablative
are associated with response to eculizumab. CR1, through binding group who received an ASCT from an unrelated or mismatched
23
C3b and C4b, enhances the decay of the C3 and C5 convertases. donor.
The density of CR1 on the surface of red cells modulates binding of In summary, ASCT should not be offered as initial therapy for
C3 fragments to the GPI-negative red cells when C5 is inhibited. most patients with classical PNH given the morbidity and mortality.
PNH patients with polymorphisms in CR1 that lead to low CR1 Exceptions are PNH patients in countries where eculizumab is not
levels (L/L genotype) are more likely to be suboptimal responders to available or patients who do not have a good response to eculizumab
eculizumab than patients with intermediate (H/L genotype) or high therapy. Patients with hypoplastic PNH and life-threatening cytope-
(H/H genotype) levels of CR1. Missense mutations in C5 (c.2654→A) nias continue to be reasonable candidates for ASCT. A myeloablative
prevent binding and therefore lead to failure to respond to eculi- conditioning regimen is not required to eradicate the PNH clone.
zumab. This rare polymorphism is found in 3.5% of the Japanese Allogeneic ASCT following nonmyeloablative conditioning regimen
population. 24 can cure PNH. Whether or not there is an advantage to one approach
over the other will require further study; however, nonmyeloablative
regimens may be preferable in young patients seeking to maintain
Thrombosis fertility or patients with moderate organ dysfunction who may not
tolerate a myeloablative regimen. Lastly, since ASCT is the only
Thrombosis is the most feared complication of PNH and is an curative therapy available for PNH, continued use and investigation
9
indication for eculizumab and anticoagulation. In patients with of this approach in selected patients is reasonable. Recent advances
acute-onset abdominal vein thrombosis, thrombolytic therapy has in mitigating GVHD such as posttransplant high-dose cyclophospha-
been successfully used. However, in some patients, thrombolytic mide may particularly be effective in PNH, but further studies are
therapy and/or anticoagulation is relatively contraindicated because required. 26