Page 751 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 751
638 Part V Red Blood Cells
simple heterozygote versus homozygote or double heterozygote) or
the presence of other genetic defects such as the presence, in trans, Laboratory Manifestations
of a defect leading to a reduced α-spectrin synthesis in some patients
with HPP. Blood Film and Laboratory Evidence of Hemolysis
The low-expression α-spectrin allele α LELY is the best-characterized
abnormality affecting spectrin content and clinical severity. Initially A careful blood smear evaluation is essential for the diagnosis of HE
a polymorphism of the αV domain, α V/41 , was identified in HE and for the classification of the disorder into the three major subtypes
patients who, when they inherited α V/41 in trans, had more severe HE outlined previously. In patients in whom elliptocytosis is the only
than expected. Subsequently an amino acid substitution of exon 46, morphologic abnormality, hemolysis is characteristically minimal or
Leu1857Val, and partial skipping of exon 46, linked to the α V/41 absent, with the exception of spherocytic elliptocytosis, in which the
polymorphism, were identified as the characteristics of the α LELY presence of round “fat” ovalocytes is associated with accelerated RBC
allele. These abnormalities are located within the site at which destruction. In patients with hemolytic forms of common HE, poi-
spectrin monomers assemble into heterodimers (the spectrin het- kilocytosis is characteristically found on the blood film. In severe
erodimer nucleation site). In vitro studies suggest that the inability forms of HE, particularly in homozygous HE, many RBCs circulate
of α-spectrin chains to assemble into the mature membrane skeleton as cell fragments, producing a marked decrease in MCV. The finding
is because of a combination of decreased αβ dimer-binding affinity of RBC fragments together with a striking microspherocytosis and
and increased proteolytic cleavage of the mutant α-spectrin chains. often only occasional elliptocytes is characteristic of HPP (Fig. 45.6).
The presence of α LELY in trans diminishes the propensity of the
otherwise normal allele to associate with the corresponding β-chain,
favoring the attachment of the elliptocytogenic α-spectrin allele. Osmotic and Thermal Fragility
Conversely, coexistence of the α-spectrin mutation in cis and the
mutation involving the α-spectrin nucleation site diminishes the Osmotic fragility is increased in HPP, in spherocytic elliptocytosis,
propensity of the mutant allele to be incorporated into the spectrin and in HE patients with poikilocytosis apparent on the peripheral
heterodimer, thereby ameliorating the clinical severity of this muta- blood film. In patients with a mild common HE without poikilocy-
tion. The α LELY allele is clinically silent by itself, even when inherited tosis on the peripheral blood film, osmotic fragility is normal.
in the homozygous state, probably because α-spectrin is normally Thermal instability of RBCs was originally reported as a charac-
synthesized in threefold to fourfold excess. teristic feature of HPP. It reflects thermal instability of the mutant
A B
C D
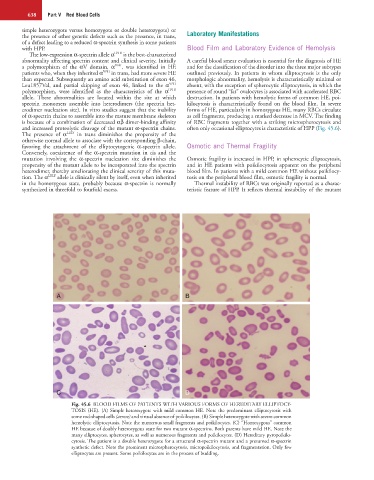
Fig. 45.6 BLOOD FILMS OF PATIENTS WITH VARIOUS FORMS OF HEREDITARY ELLIPTOCY-
TOSIS (HE). (A) Simple heterozygote with mild common HE. Note the predominant elliptocytosis with
some rod-shaped cells (arrow) and virtual absence of poikilocytes. (B) Simple heterozygote with severe common
hemolytic elliptocytosis. Note the numerous small fragments and poikilocytes. (C) “Homozygous” common
HE because of doubly heterozygous state for two mutant α-spectrins. Both parents have mild HE. Note the
many elliptocytes, spherocytes, as well as numerous fragments and poikilocytes. (D) Hereditary pyropoikilo-
cytosis. The patient is a double heterozygote for a structural α-spectrin mutant and a presumed α-spectrin
synthetic defect. Note the prominent microspherocytosis, micropoikilocytosis, and fragmentation. Only few
elliptocytes are present. Some poikilocytes are in the process of budding.