Page 826 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 826
712 Part VI Non-Malignant Leukocytes
Bone marrow Spleen/lymph node
BTK
RAG1 IGM Marginal zone sIgM
RAG2 CD79A/B Foll-B
IGLL sIgD
Pre-BCR sIgM
HSC Pro-B Pre-B immB CD40- Follicular
CD40L ActB zone CD40L
CD40
CD4 IL-21 AID
UNG
sIgG
sIgA CSR
sIgM SHM
immB
sIgM sIgE sIgA
sIgG
MZ-B
sIgE
memB PC
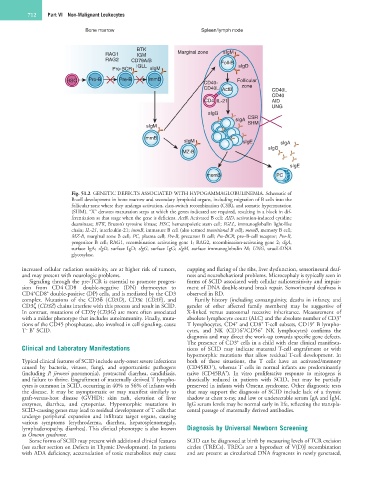
Fig. 51.2 GENETIC DEFECTS ASSOCIATED WITH HYPOGAMMAGLOBULINEMIA. Schematic of
B-cell development in bone marrow and secondary lymphoid organs, including migration of B cells into the
follicular zone where they undergo activation, class-switch recombination (CSR), and somatic hypermutation
(SHM). “X” denotes maturation steps at which the genes indicated are required, resulting in a block in dif-
ferentiation at that stage when the gene is deficient. ActB, Activated B cell; AID, activation-induced cytidine
deaminase; BTK, Bruton’s tyrosine kinase; HSC, hematopoietic stem cell; IGLL, immunoglobulin light-like
chain; IL-21, interleukin-21; immB, immature B cell (also termed transitional B cell); memB, memory B cell;
MZ-B, marginal zone B cell; PC, plasma cell; Pre-B, precursor B cell; Pre-BCR, pre–B-cell receptor; Pro-B,
progenitor B cell; RAG1, recombination activating gene 1; RAG2, recombination-activating gene 2; sIgA,
surface IgA; sIgD, surface IgD; sIgG, surface IgG; sIgM, surface immunoglobulin M; UNG, uracil-DNA
glycosylase.
increased cellular radiation sensitivity, are at higher risk of tumors, cupping and flaring of the ribs, liver dysfunction, sensorineural deaf-
and may present with neurologic problems. ness and neurobehavioral problems. Microcephaly is typically seen in
Signaling through the pre-TCR is essential to promote progres- forms of SCID associated with cellular radiosensitivity and impair-
–
–
sion from CD4 CD8 double-negative (DN) thymocytes to ment of DNA double-strand break repair. Sensorineural deafness is
+
+
CD4 CD8 double-positive (DP) cells, and is mediated by the CD3 observed in RD.
complex. Mutations of the CD3δ (CD3D), CD3ε (CD3E), and Family history (including consanguinity, deaths in infancy, and
CD3ζ (CD3Z) chains interfere with this process and result in SCID. gender of other affected family members) may be suggestive of
In contrast, mutations of CD3γ (CD3G) are more often associated X-linked versus autosomal recessive inheritance. Measurement of
+
with a milder phenotype that includes autoimmunity. Finally, muta- absolute lymphocyte count (ALC) and the absolute number of CD3
+
+
+
tions of the CD45 phosphatase, also involved in cell signaling, cause T lymphocytes, CD4 and CD8 T-cell subsets, CD19 B lympho-
+
+
+
–
T B SCID. cytes, and NK (CD16 /CD56 NK lymphocytes) confirms the
diagnosis and may direct the work-up towards specific gene defects.
+
The presence of CD3 cells in a child with clear clinical manifesta-
Clinical and Laboratory Manifestations tions of SCID may indicate maternal T-cell engraftment or with
hypomorphic mutations that allow residual T-cell development. In
Typical clinical features of SCID include early-onset severe infections both of these situations, the T cells have an activated/memory
+
caused by bacteria, viruses, fungi, and opportunistic pathogens (CD45RO ), whereas T cells in normal infants are predominantly
+
(including P. jiroveci pneumonia), protracted diarrhea, candidiasis, naive (CD45RA ). In vitro proliferative response to mitogens is
and failure to thrive. Engraftment of maternally derived T lympho- drastically reduced in patients with SCID, but may be partially
cytes is common in SCID, occurring in 40% to 56% of infants with preserved in infants with Omenn syndrome. Other diagnostic tests
the disease. It may be asymptomatic or may manifest similarly to that may support the diagnosis of SCID include lack of a thymic
graft-versus-host disease (GVHD): skin rash, elevation of liver shadow at chest x-ray, and low or undetectable serum IgA and IgM.
enzymes, diarrhea, and cytopenias. Hypomorphic mutations in IgG serum levels may be normal early in life, reflecting the transpla-
SCID-causing genes may lead to residual development of T cells that cental passage of maternally derived antibodies.
undergo peripheral expansion and infiltrate target organs, causing
various symptoms (erythroderma, diarrhea, hepatosplenomegaly,
lymphadenopathy, diarrhea). This clinical phenotype is also known Diagnosis by Universal Newborn Screening
as Omenn syndrome.
Some forms of SCID may present with additional clinical features SCID can be diagnosed at birth by measuring levels of TCR excision
(see earlier section on Defects in Thymic Development). In patients circles (TRECs). TRECs are a byproduct of V(D)J recombination
with ADA deficiency, accumulation of toxic metabolites may cause and are present as circularized DNA fragments in newly generated,