Page 825 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 825
Chapter 51 Congenital Disorders of Lymphocyte Function 711
ADA, PNP γc, IL7R, JAK3
NK
NKp
Thymus
CD3δ,ε,ζ
T/NKp TRAC, CD45 CD4 CD4
AK2
HSC CLP DN DP
CD8 CD8
FOXN1
Bp B
Myeloid RAG1/2, Artemis, DNA-PKcs
progenitor LIG4, Cernunnos
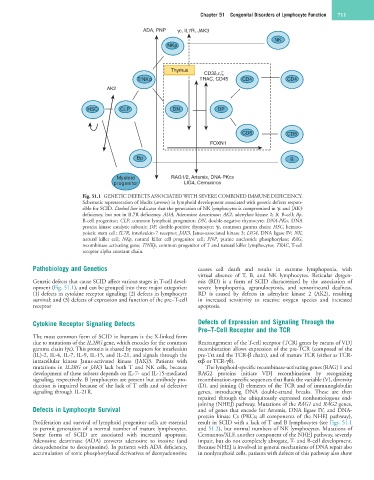
Fig. 51.1 GENETIC DEFECTS ASSOCIATED WITH SEVERE COMBINED IMMUNE DEFICIENCY.
Schematic representation of blocks (arrows) in lymphoid development associated with genetic defects respon-
sible for SCID. Dashed line indicates that the generation of NK lymphocytes is compromised in γc and JAK3
deficiency, but not in IL7R deficiency. ADA, Adenosine deaminase; AK2, adenylate kinase 2; B, B-cell; Bp,
B-cell progenitor; CLP, common lymphoid progenitor; DN, double-negative thymocyte; DNA-PKcs, DNA
protein kinase catalytic subunit; DP, double-positive thymocyte; γc, common gamma chain; HSC, hemato-
poietic stem cell; IL7R, interleukin-7 receptor; JAK3, Janus-associated kinase 3; LIG4, DNA ligase IV; NK,
natural killer cell; NKp, natural killer cell progenitor cell; PNP, purine nucleoside phosphorylase; RAG,
recombinase activating gene; T/NKp, common progenitor of T and natural killer lymphocytes; TRAC, T-cell
receptor alpha constant chain.
Pathobiology and Genetics causes cell death and results in extreme lymphopenia, with
virtual absence of T, B, and NK lymphocytes. Reticular dysgen-
Genetic defects that cause SCID affect various stages in T-cell devel- esis (RD) is a form of SCID characterized by the association of
opment (Fig. 51.1), and can be grouped into three major categories: severe lymphopenia, agranulocytosis, and sensorineural deafness.
(1) defects in cytokine receptor signaling; (2) defects in lymphocyte RD is caused by defects in adenylate kinase 2 (AK2), resulting
survival; and (3) defects of expression and function of the pre–T-cell in increased sensitivity to reactive oxygen species and increased
receptor apoptosis.
Cytokine Receptor Signaling Defects Defects of Expression and Signaling Through the
Pre–T-Cell Receptor and the TCR
The most common form of SCID in humans is the X-linked form
due to mutations of the IL2RG gene, which encodes for the common Rearrangement of the T-cell receptor (TCR) genes by means of VDJ
gamma chain (γc). This protein is shared by receptors for interleukin recombination allows expression of the pre-TCR (composed of the
(IL)-2, IL-4, IL-7, IL-9, IL-15, and IL-21, and signals through the pre-Tα and the TCR-β chain), and of mature TCR (either as TCR-
intracellular kinase Janus-activated kinase (JAK)3. Patients with αβ or TCR-γδ).
mutations in IL2RG or JAK3 lack both T and NK cells, because The lymphoid-specific recombinase-activating genes (RAG) 1 and
development of these subsets depends on IL-7- and IL-15-mediated RAG2 proteins initiate VDJ recombination by recognizing
signaling, respectively. B lymphocytes are present but antibody pro- recombination-specific sequences that flank the variable (V), diversity
duction is impaired because of the lack of T cells and of defective (D), and joining (J) elements of the TCR and of immunoglobulin
signaling through IL-21R. genes, introducing DNA double-strand breaks. These are then
repaired through the ubiquitously expressed nonhomologous end-
joining (NHEJ) pathway. Mutations of the RAG1 and RAG2 genes,
Defects in Lymphocyte Survival and of genes that encode for Artemis, DNA ligase IV, and DNA-
protein kinase Cs (PKCs; all components of the NHEJ pathway),
Proliferation and survival of lymphoid progenitor cells are essential result in SCID with a lack of T and B lymphocytes (see Figs. 51.1
to permit generation of a normal number of mature lymphocytes. and 51.2), but normal numbers of NK lymphocytes. Mutations of
Some forms of SCID are associated with increased apoptosis. Cernunnos/XLF, another component of the NHEJ pathway, severely
Adenosine deaminase (ADA) converts adenosine to inosine (and impair, but do not completely abrogate, T- and B-cell development.
deoxyadenosine to deoxyinosine). In patients with ADA deficiency, Because NHEJ is involved in general mechanisms of DNA repair also
accumulation of toxic phosphorylated derivatives of deoxyadenosine in nonlymphoid cells, patients with defects of this pathway also show