Page 997 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 997
880 Part VII Hematologic Malignancies
A B
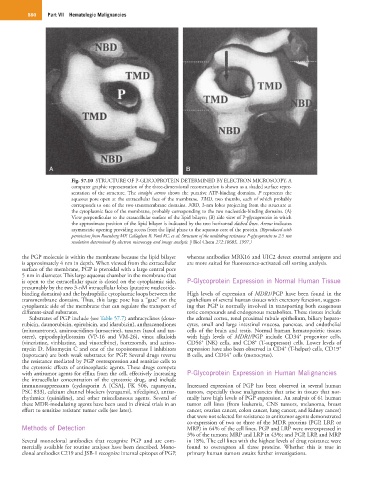
Fig. 57.10 STRUCTURE OF P-GLYCOPROTEIN DETERMINED BY ELECTRON MICROSCOPY. A
computer graphic representation of the three-dimensional reconstruction is shown as a shaded surface repre-
sentation of the structure. The straight arrow shows the putative ATP-binding domains. P represents the
aqueous pore open at the extracellular face of the membrane. TMD, two thumbs, each of which probably
corresponds to one of the two transmembrane domains. NBD, 3-nm lobes projecting from the structure at
the cytoplasmic face of the membrane, probably corresponding to the two nucleotide-binding domains. (A)
View perpendicular to the extracellular surface of the lipid bilayer; (B) side view of P-glycoprotein in which
the approximate position of the lipid bilayer is indicated by the two horizontal dashed lines. Arrow indicates
asymmetric opening providing access from the lipid phase to the aqueous core of the protein. (Reproduced with
permission from Rosenberg MF, Callaghan R, Ford RC, et al: Structure of the multidrug resistance P-glycoprotein to 2.5 nm
resolution determined by electron microscopy and image analysis. J Biol Chem 272:10685, 1997.)
the PGP molecule is within the membrane because the lipid bilayer whereas antibodies MRK16 and UIC2 detect external antigens and
is approximately 4 nm in depth. When viewed from the extracellular are more suited for fluorescence-activated cell sorting analysis.
surface of the membrane, PGP is pteroidal with a large central pore
5 nm in diameter. This large aqueous chamber in the membrane that
is open to the extracellular space is closed on the cytoplasmic side, P-Glycoprotein Expression in Normal Human Tissue
presumably by the two 3-nM intracellular lobes (putative nucleotide-
binding domains) and the hydrophilic cytoplasmic loops between the High levels of expression of MDR1/PGP have been found in the
transmembrane domains. Thus, this large pore has a “gate” on the epithelium of several human tissues with excretory function, suggest-
cytoplasmic side of the membrane that can regulate the transport of ing that PGP is normally involved in transporting both exogenous
different-sized substrates. toxic compounds and endogenous metabolites. These tissues include
Substrates of PGP include (see Table 57.7) anthracyclines (doxo- the adrenal cortex, renal proximal tubule epithelium, biliary hepato-
rubicin, daunorubicin, epirubicin, and idarubicin), anthracenediones cytes, small and large intestinal mucosa, pancreas, and endothelial
(mitoxantrone), aminoacridines (amsacrine), taxanes (taxol and tax- cells of the brain and testis. Normal human hematopoietic tissues
+
otere), epipodophyllotoxins (VP-16 and VM-26), vinca alkaloids with high levels of MDR1/PGP include CD34 progenitor cells,
+
+
(vincristine, vinblastine, and vinorelbine), bortezomib, and actino- CD56 (NK) cells, and CD8 (T-suppressor) cells. Lower levels of
+
+
mycin D. Mitomycin C and one of the topoisomerase I inhibitors expression have also been observed in CD4 (T-helper) cells, CD19
+
(topotecan) are both weak substrates for PGP. Several drugs reverse B cells, and CD14 cells (monocytes).
the resistance mediated by PGP overexpression and sensitize cells to
the cytotoxic effects of antineoplastic agents. These drugs compete
with antitumor agents for efflux from the cell, effectively increasing P-Glycoprotein Expression in Human Malignancies
the intracellular concentration of the cytotoxic drug, and include
immunosuppressants (cyclosporin A [CSA], FK 506, rapamycin, Increased expression of PGP has been observed in several human
PSC 833), calcium channel blockers (verapamil, nifedipine), antiar- tumors, especially those malignancies that arise in tissues that nor-
rhythmics (quinidine), and other miscellaneous agents. Several of mally have high levels of PGP expression. An analysis of 61 human
these MDR-modulating agents have been used in clinical trials in an tumor cell lines (from leukemia, CNS tumors, melanoma, breast
effort to sensitize resistant tumor cells (see later). cancer, ovarian cancer, colon cancer, lung cancer, and kidney cancer)
that were not selected for resistance to antitumor agents demonstrated
co-expression of two or three of the MDR proteins (PGP, LRP, or
Methods of Detection MRP) in 64% of the cell lines. PGP and LRP were overexpressed in
3% of the tumors; MRP and LRP in 43%; and PGP, LRP, and MRP
Several monoclonal antibodies that recognize PGP and are com- in 18%. The cell lines with the highest levels of drug resistance were
mercially available for routine analyses have been described. Mono- found to overexpress all three proteins. Whether this is true in
clonal antibodies C219 and JSB-1 recognize internal epitopes of PGP, primary human tumors awaits further investigations.