Page 1032 - Williams Hematology ( PDFDrive )
P. 1032
1006 Part VII: Neutrophils, Eosinophils, Basophils, and Mast Cells Chapter 66: Disorders of Neutrophil Function 1007
2 3 4
1
Basal membrane
Stroma
Chemokines
MΦ 6
Bacteria
5
7
PMN
Chemokines
Prim.
gr. Sec. gr.
T cells
Tert. gr.
8
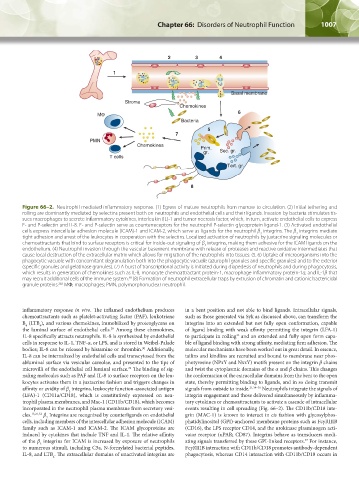
Figure 66–2. Neutrophil-mediated inflammatory response. (1) Egress of mature neutrophils from marrow to circulation. (2) Initial tethering and
rolling are dominantly mediated by selectins present both on neutrophils and endothelial cells and their ligands. Invasion by bacteria stimulates tis-
sues macrophages to secrete inflammatory cytokines, interleukin (IL)-1 and tumor necrosis factor, which, in turn, activate endothelial cells to express
E- and P-selectin and IL-8. E- and P-selectin serve as counterreceptors for the neutrophil P-selectin glycoprotein ligand-1. (3) Activated endothelial
cells express intercellular adhesion molecule (ICAM)-1 and ICAM-2, which serve as ligands for the neutrophil β integrins. The β integrins mediate
2
2
tight adhesion and arrest of the leukocytes in cooperation with the selectins. Localized activation of neutrophils by juxtacrine signaling molecules or
chemoattractants that bind to surface receptors is critical for inside-out signaling of β integrins, making them adhesive for the ICAM ligands on the
2
endothelium. (4) Neutrophil invasion through the vascular basement membrane with release of proteases and reactive oxidative intermediates that
cause local destruction of the extracellular matrix which allows for migration of the neutrophils into tissues. (5, 6) Uptake of microorganisms into the
phagocytic vacuole with concomitant degranulation both into the phagocytic vacuole (azurophil granules and specific granules) and to the exterior
(specific granules and gelatinase granules). (7) A burst of transcriptional activity is initiated during diapedesis of neutrophils and during phagocytosis,
which results in generation of chemokines such as IL-8, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and IL-1β that
may recruit additional cells of the immune system. (8) Formation of neutrophil extracellular traps by extrusion of chromatin and cationic bactericidal
58
granule proteins. MΦ, macrophages; PMN, polymorphonuclear neutrophil.
262
inflammatory response in vivo. The inflamed endothelium produces in a bent position and not able to bind ligands. Intracellular signals,
chemoattractants such as platelet-activating factor (PAF), leukotriene such as those generated via Syk as discussed above, can transform the
B (LTB ), and various chemokines, immobilized by proteoglycans on integrins into an extended but not fully open conformation, capable
4
4
the luminal surface of endothelial cells. Among these chemokines, of ligand binding with weak affinity permitting the integrin (LFA-1)
29
IL-8 specifically attracts neutrophils. IL-8 is synthesized by endothelial to participate in rolling and an extended and fully open form capa-
33
cells in response to IL-1, TNF-α, or LPS, and is stored in Weibel-Palade ble of ligand binding with strong affinity, mediating firm adhesion. The
bodies; IL-8 can be released by histamine or thrombin. Additionally, molecular mechanisms have been worked out in great detail. In essence,
30
IL-8 can be internalized by endothelial cells and transcytosed from the tailins and kindlins are recruited and bound to membrane near phos-
abluminal surface via vesicular caveolae, and presented to the tips of photyrosine (NPxY and NxxY) motifs present on the integrin β chains
microvilli of the endothelial cell luminal surface. The binding of sig- and twist the cytoplasmic domains of the α and β chains. This changes
31
naling molecules such as PAF and IL-8 to surface receptors on the leu- the conformation of the extracellular domains from the bent to the open
kocytes activates them in a juxtacrine fashion and triggers changes in state, thereby permitting binding to ligands, and in so doing transmit
affinity or avidity of β integrins, leukocyte function-associated antigen signals from outside to inside. 21,34–36 Neutrophils integrate the signals of
2
(LFA)-1 (CD11a/CD18), which is constitutively expressed on neu- integrin engagement and those delivered simultaneously by inflamma-
trophil plasma membranes, and Mac-1 (CD11b/CD18), which becomes tory cytokines or chemoattractants to activate a cascade of intracellular
incorporated in the neutrophil plasma membrane from secretory vesi- events resulting in cell spreading (Fig. 66–2). The CD11b/CD18 inte-
cles. 13,21,32 β Integrins are recognized by counterligands on endothelial grin (MAC-1) is known to interact in cis fashion with glycosylphos-
2
cells, including members of the intercellular adhesion molecule (ICAM) phatidylinositol (GPI)-anchored membrane proteins such as FcγRIIIB
family such as ICAM-1 and ICAM-2. The ICAM glycoproteins are (CD16), the LPS receptor CD14, and the urokinase plasminogen acti-
induced by cytokines that include TNF and IL-1. The relative affinity vator receptor (uPAR; CD87). Integrins behave as transducers medi-
of the β integrins for ICAM is increased by exposure of neutrophils ating signals transferred by these GPI-linked receptors. For instance,
37
2
to numerous stimuli, including C5a, N-formylated bacterial peptides, FcγRIIIB interaction with CD11b/CD18 promotes antibody-dependent
IL-8, and LTB . The extracellular domains of unactivated integrins are phagocytosis, whereas CD14 interaction with CD11b/CD18 occurs in
4
Kaushansky_chapter 66_p1005-1042.indd 1007 9/21/15 10:47 AM