Page 1210 - Williams Hematology ( PDFDrive )
P. 1210
1184 Part IX: Lymphocytes and Plasma Cells Chapter 76: Functions of T Lymphocytes: T-cell Receptors for Antigen 1185
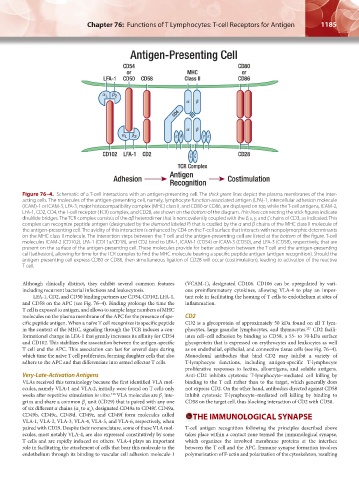
Figure 76–4. Schematic of a T-cell interactions with an antigen-presenting cell. The thick green lines depict the plasma membranes of the inter-
acting cells. The molecules of the antigen-presenting cell, namely, lymphocyte function-associated antigen (LFA)-1, intercellular adhesion molecule
(ICAM)-1 or ICAM-3, LFA-3, major histocompatibility complex (MHC) class II, and CD80 or CD86, are displayed on top, while the T-cell antigens, ICAM-2,
LFA-1, CD2, CD4, the T-cell receptor (TCR) complex, and CD28, are shown on the bottom of the diagram. Thin lines connecting the stick figures indicate
disulfide bridges. The TCR complex consists of the αβ heterodimer that is noncovalently coupled with the δ, ε, γ, and ζ chains of CD3, as indicated. This
complex can recognize peptide antigen (designated by the diamond labeled P) that is cradled by the α and β chains of the MHC class II molecule of
the antigen-presenting cell. The avidity of this interaction is enhanced by CD4 on the T-cell surface that interacts with nonpolymorphic determinants
on the MHC class II molecule. The interaction steps between the T cell and the antigen-presenting cell are listed at the bottom of the figure. T-cell
molecules ICAM-2 (CD102), LFA-1 (CD11a/CD18), and CD2 bind to LFA-1, ICAM-1 (CD54) or ICAM-3 (CD50), and LFA-3 (CD58), respectively, that are
present on the surface of the antigen-presenting cell. These molecules provide for better adhesion between the T cell and the antigen-presenting
cell (adhesion), allowing for time for the TCR complex to find the MHC molecule bearing a specific peptide antigen (antigen recognition). Should the
antigen- presenting cell express CD80 or CD86, then simultaneous ligation of CD28 will occur (costimulation), leading to activation of the reactive
T cell.
Although clinically distinct, they exhibit several common features (VCAM-1), designated CD106. CD106 can be upregulated by vari-
including recurrent bacterial infections and leukocytosis. ous proinflammatory cytokines, allowing VLA-4 to play an impor-
LFA-1, CD2, and CD50 binding partners are CD54, CD102, LFA-1, tant role in facilitating the homing of T cells to endothelium at sites of
and CD58 on the APC (see Fig. 76–4). Binding prolongs the time the inflammation.
T cell is exposed to antigen, and allows to sample large numbers of MHC
molecules on the plasma membrane of the APC for the presence of spe- CD2
cific peptide antigen. When a naïve T cell recognizes its specific peptide CD2 is a glycoprotein of approximately 50 kDa found on all T lym-
in the context of the MHC, signaling through the TCR induces a con- phocytes, large granular lymphocytes, and thymocytes. CD2 facili-
127
formational change in LFA-1 that greatly increases its affinity for CD54 tates cell–cell adhesion by binding to CD58, a 55- to 70-kDa surface
and CD102. This stabilizes the association between the antigen-specific glycoprotein that is expressed on erythrocytes and leukocytes as well
T cell and the APC. This association can last for several days during as on endothelial, epithelial, and connective tissue cells (see Fig. 76–4).
which time the naïve T cell proliferates, forming daughter cells that also Monoclonal antibodies that bind CD2 may inhibit a variety of
adhere to the APC and that differentiate into armed effector T cells. T-lymphocyte functions, including antigen-specific T-lymphocyte
proliferative responses to lectins, alloantigens, and soluble antigens.
Very-Late-Activation Antigens Anti-CD2 inhibits cytotoxic T-lymphocyte–mediated cell killing by
VLAs received this terminology because the first identified VLA mol- binding to the T cell rather than to the target, which generally does
ecules, namely VLA-1 and VLA-2, initially were found on T cells only not express CD2. On the other hand, antibodies directed against CD58
weeks after repetitive stimulation in vitro. VLA molecules are β inte- inhibit cytotoxic T-lymphocyte–mediated cell killing by binding to
126
1
grins and share a common β unit (CD29) that is paired with any one CD58 on the target cell, thus blocking interaction of CD2 with CD58.
1
of six different α chains (α to α ), designated CD49a to CD49f. CD49a,
6
1
CD49b, CD49c, CD49d, CD49e, and CD49f form molecules called THE IMMUNOLOGICAL SYNAPSE
VLA-1, VLA-2, VLA-3, VLA-4, VLA-5, and VLA-6, respectively, when
paired with CD29. Despite their nomenclature, some of these VLA mol- T-cell antigen recognition following the principles described above
ecules, most notably VLA-4, are also expressed constitutively by some takes place within a contact zone termed the immunological synapse,
T cells and are rapidly induced on others. VLA-4 plays an important which organizes the involved membrane proteins at the interface
role in facilitating the attachment of cells that bear this molecule to the between the T cell and the APC. Immune synapse formation involves
endothelium through its binding to vascular cell adhesion molecule-1 polymerization of F-actin and polarization of the cytoskeleton, resulting
Kaushansky_chapter 76_p1175-1188.indd 1185 9/17/15 4:01 PM