Page 1372 - Williams Hematology ( PDFDrive )
P. 1372
1346 Part X: Malignant Myeloid Diseases Chapter 87: Myelodysplastic Syndromes 1347
A B C
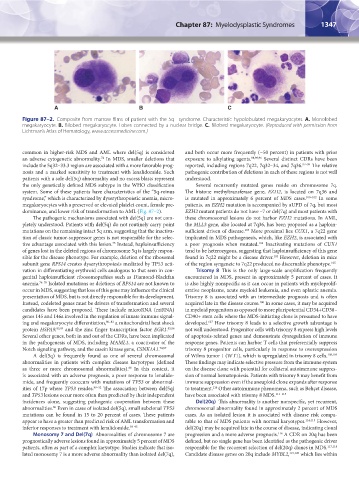
Figure 87–2. Composite from marrow films of patient with the 5q– syndrome. Characteristic hypolobulated megakaryocytes. A. Monolobed
megakaryocyte. B. Bilobed megakaryocyte. Lobes connected by a nuclear bridge. C. Bilobed megakaryocyte. (Reproduced with permission from
Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
common in higher-risk MDS and AML where del(5q) is considered and both occur more frequently (~50 percent) in patients with prior
an adverse cytogenetic abnormality. In MDS, smaller deletions that exposure to alkylating agents. 88,89,96 Several distinct CDRs have been
74
include the 5q32–33.3 region are associated with a more favorable prog- reported, including regions 7q22, 7q32–34, and 7q36. 97–99 The relative
nosis and a marked sensitivity to treatment with lenalidomide. Such pathogenic contribution of deletions in each of these regions is not well
patients with a sole del(5q) abnormality and no excess blasts represent understood.
the only genetically defined MDS subtype in the WHO classification Several recurrently mutated genes reside on chromosome 7q.
system. Some of these patients have characteristics of the “5q-minus The histone methyltransferase gene, EZH2, is located on 7q36 and
syndrome,” which is characterized by dyserythropoietic anemia, micro- is mutated in approximately 6 percent of MDS cases. 100–102 In some
megakaryocytes with a preserved or elevated platelet count, female pre- patients, an EZH2 mutation is accompanied by aUPD of 7q, but most
dominance, and lower risk of transformation to AML (Fig. 87–2). EZH2 mutant patients do not have –7 or del(7q) and most patients with
The pathogenic mechanisms associated with del(5q) are not com- these chromosomal lesions do not harbor EZH2 mutations. In AML,
pletely understood. Patients with del(5q) do not routinely carry point the MLL3 gene, also located at 7q36, has been proposed as a haploin-
mutations on the remaining intact 5q arm, suggesting that the inactiva- sufficient driven of disease. More proximal lies CUX1, a 7q22 gene
103
tion of classic tumor-suppressor genes is not responsible for the selec- implicated in MDS pathogenesis, which, like EZH2, is associated with
tive advantage associated with this lesion. Instead, haploinsufficiency a poor prognosis when mutated. Inactivating mutations of CUX1
104
75
of genes lost in the deleted regions of chromosome 5q is largely respon- tend to be heterozygous, suggesting that haploinsufficiency of this gene
sible for the disease phenotype. For example, deletion of the ribosomal found in 7q22 might be a disease driver. However, deletion in mice
104
subunit gene RPS14 creates dyserythropoiesis mediated by TP53 acti- of the region syngeneic to 7q22 produced no discernable phenotype. 105
vation in differentiating erythroid cells analogous to that seen in con- Trisomy 8 This is the only large-scale amplification frequently
genital haploinsufficient ribosomopathies such as Diamond-Blackfan encountered in MDS, present in approximately 5 percent of cases. It
anemia. 76–79 Isolated mutations or deletions of RPS14 are not known to is also highly nonspecific as it can occur in patients with myeloprolif-
occur in MDS, suggesting that loss of this gene may influence the clinical erative neoplasms, acute myeloid leukemia, and even aplastic anemia.
presentation of MDS, but is not directly responsible for its development. Trisomy 8 is associated with an intermediate prognosis and is often
Instead, codeleted genes must be drivers of transformation and several acquired late in the disease course. In some cases, it may be acquired
106
candidates have been proposed. These include microRNA (miRNA) in myeloid progenitors as opposed to more pluripotential CD34+CD38–
genes 145 and 146a involved in the regulation of innate immune signal- CD90+ stem cells where the MDS-initiating clone is presumed to have
ing and megakaryocyte differentiation, 80–82 a mitochondrial heat shock developed. How trisomy 8 leads to a selective growth advantage is
107
protein HSPA9, 83,84 and the zinc finger transcription factor EGR1. 85,86 not well understood. Progenitor cells with trisomy 8 express high levels
Several other genes, both in and out of the CDRs, have been implicated of apoptosis-related genes and demonstrate dysregulation of immune
in the pathogenesis of MDS, including MAML1, a coactivator of the response genes. Patients can harbor T cells that preferentially suppress
Notch signaling pathway, and the casein kinase gene, CSNK1A1. 74,87 trisomy 8 progenitor cells, particularly in response to overexpression
A del(5q) is frequently found as one of several chromosomal of Wilms tumor 1 (WT1), which is upregulated in trisomy 8 cells. 108,109
abnormalities in patients with complex disease karyotypes (defined These findings may indicate selective pressure from the immune system
as three or more chromosomal abnormalities). In this context, it on the disease clone with potential for collateral autoimmune suppres-
88
is associated with an adverse prognosis, a poor response to lenalido- sion of normal hematopoiesis. Patients with trisomy 8 may benefit from
mide, and frequently cooccurs with mutations of TP53 or abnormal- immune suppression even if the aneuploid clone expands after response
ities of 17p where TP53 resides. 89–92 The association between del(5q) to treatment. Other autoimmune phenomena, such as Behçet disease,
110
and TP53 lesions occur more often than predicted by their independent have been associated with trisomy 8 MDS. 111–113
incidences alone, suggesting pathogenic cooperation between these Del(20q) This abnormality is another nonspecific, yet recurrent,
abnormalities. Even in cases of isolated del(5q), small subclonal TP53 chromosomal abnormality found in approximately 2 percent of MDS
86
mutations can be found in 15 to 20 percent of cases. These patients cases. As an isolated lesion it is associated with disease risk compa-
appear to have a greater than predicted risk of AML transformation and rable to that of MDS patients with normal karyotypes. 114,115 However,
inferior responses to treatment with lenalidomide. 93–95 del(20q) may be acquired late in the course of disease, indicating clonal
Monosomy 7 and Del(7q) Abnormalities of chromosome 7 are progression and a more adverse prognosis. A CDR on 20q has been
116
prognostically adverse lesions found in approximately 5 percent of MDS defined, but no single gene has been identified as the pathogenic driver
patients, often as part of a complex karyotype. Studies indicate that iso- responsible for the recurrent selection of del(20q) clones in MDS. 117,118
lated monosomy 7 is a more adverse abnormality than isolated del(7q), Candidate disease genes on 20q include MYBL2, 119,120 which lies within
Kaushansky_chapter 87_p1341-1372.indd 1347 9/21/15 11:05 AM