Page 1400 - Williams Hematology ( PDFDrive )
P. 1400
1374 Part X: Malignant Myeloid Diseases Chapter 88: Acute Myelogenous Leukemia 1375
probability of occurrence in each chronic myeloid neoplasm (Chap. 83). The blood cells of more than 2 percent of individuals (5 to 6 percent
The frequency of clonal progression to AML is enhanced by radiation of people older than 70 years) contain mutations that may represent
or chemotherapy in patients with polycythemia vera (Chap. 84) or premalignant events that can cause clonal hematopoietic expansion.
essential thrombocythemia (Chap. 85). Although some refer to this as These events may, in part, explain the age-dependent incidence of AML
29
secondary AML, it should be called clonally evolved AML (ceAML) to (Fig. 88–1). 29a
distinguish it from secondary AML that results from radiation or che-
motherapy given to patients who do not have a precedent clonal mye- PREDISPOSING DISEASES
loid disease. In the population of patients with preceding clonal myeloid
neoplasms, a myeloid leukemic clone already exists and is not induced Patients who develop AML may have an antecedent predisposing non-
secondarily. Evolution to AML represents the natural history of the neo- myeloid disease, such as aplastic anemia (poly- or oligoclonal T-cell
plasm, albeit sometimes accelerated by various external mutagens. disorder), myeloma (monoclonal B-cell disorder), 30,31 or, rarely, AIDS
(HIV-induced polyclonal T-cell disorder). An association between
32
Langerhans cell histiocytosis, immune thyroid diseases, and familial
AGING AND ACUTE MYELOGENOUS polyendocrine disorder and AML has been reported. 33–36 A number of
inherited conditions carry an increased risk of AML (see Table 88–1).
37–
LEUKEMIA–RELEVANT SOMATIC MUTATIONS 80 In the inherited syndromes, at least several pathogenetic types of
Very low copy number gene mutations characteristic of leukemia or gene alterations are represented: (1) DNA repair defects, for exam-
lymphoma have been detected in the blood of healthy individuals. An ple, Fanconi anemia; (2) susceptibility genes favoring a second muta-
analysis of blood cell DNA sequence data has identified 77 blood cell– tion, for example, familial platelet syndrome; (3) tumor-suppressor
specific mutations in cancer-associated genes, the majority being asso- defects, for example, dyskeratosis congenita; and (4) unknown mech-
ciated with advanced age. A large majority of these mutations were from anisms, for example, ataxia-pancytopenia (See Tables 35-8 and 35-9 in
19 leukemia and/or lymphoma-associated genes, and nine were recur- Chap. 35 for further details of each pathogenetic process). There is
rently mutated (DNMT3A, TET2, JAK2, ASXL1, TP53, GNAS, PPM1D, evidence from central registry studies that any disorder that results in
BCORL1, and SF3B1). Additional mutations were found in a very small chronic immune stimulation, such as infection or autoimmune diseases
fraction of blood cells. Comparison of these findings to mutations in may be associated with AML and MDS. The prevalence of essential
81
hematologic malignancies identified other recurrently mutated genes. monoclonal gammopathy is not increased in AML patients. 82
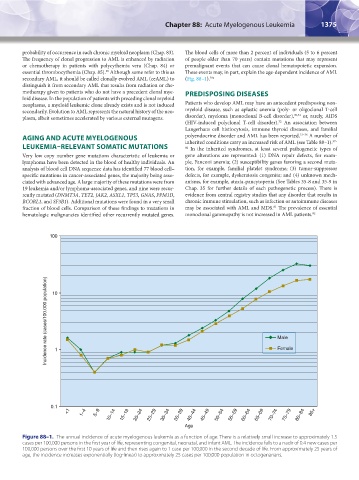
100
Incidence rate (cases/100,000 population) 10 Male
Female
1
0.1
<1 1–4 5–9 10–14 15–19 20–24 25–29 30–34 35–39 40–44 45–49 50–54 55–59 60–64 65–69 70–74 75–79 80–84 85+
Age
Figure 88–1. The annual incidence of acute myelogenous leukemia as a function of age. There is a relatively small increase to approximately 1.5
cases per 100,000 persons in the first year of life, representing congenital, neonatal, and infant AML. The incidence falls to a nadir of 0.4 new cases per
100,000 persons over the first 10 years of life and then rises again to 1 case per 100,000 in the second decade of life. From approximately 25 years of
age, the incidence increases exponentially (log-linear) to approximately 25 cases per 100,000 population in octogenarians.
Kaushansky_chapter 88_p1373-1436.indd 1375 9/21/15 11:00 AM