Page 158 - Williams Hematology ( PDFDrive )
P. 158
132 Part III: Epochal Hematology Chapter 9: Hematology in Older Persons 133
in several organs, but after birth this function is subsumed by the mar- senescence and that such changes are responsible for the development of
row. 142–148 The process of embryonic and fetal hematopoiesis is described immune senescence, as well as the increased occurrence of age-associ-
in Chap. 7. Hematopoietic cells appear in the medullary cavities of bone ated diseases such as myelodysplasia and leukemia. Thus, the process of
around 14 weeks of gestation, and by birth the marrow is the primary “immunosenescence,” as it affects the innate and adaptive immune sys-
149
site of hematopoiesis. tem, may result from HSC aging. For example, an age-related decrease
Unlike the commonly held notion that stem cell compartments in the provision of B-cell precursors may be the result of HSC aging. 148
diminish either in number or function with age ultimately resulting in
an inability to meet homeostatic demands, age-related hematopoietic
stem cell (HSC) changes appear to be an exception, at least for murine MARROW DURING ADULT LIFE
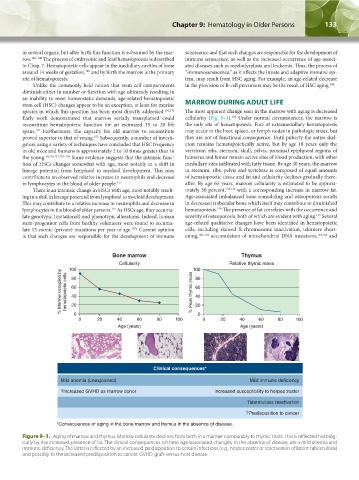
species in which this question has been most directly addressed. 150,151 The most apparent change seen in the marrow with aging is decreased
135
Early work demonstrated that marrow serially transplanted could cellularity (Fig. 9–1). Under normal circumstances, the marrow is
reconstitute hematopoietic function for an estimated 15 to 20 life the only site of hematopoiesis. Foci of extramedullary hematopoiesis
152
spans. Furthermore, the capacity for old marrow to reconstitute may occur in the liver, spleen, or lymph nodes in pathologic states, but
proved superior to that of young. Subsequently, a number of investi- they are not of functional consequence. Until puberty the entire skel-
153
gators using a variety of techniques have concluded that HSC frequency eton remains hematopoietically active, but by age 18 years only the
in old mice and humans is approximately 2 to 10 times greater than in vertebrae, ribs, sternum, skull, pelvis, proximal epiphyseal regions of
the young. 16,150,151,154–156 Some evidence suggests that the intrinsic func- humerus and femur remain active sites of blood production, with other
tion of HSCs changes somewhat with age, most notably in a shift in medullary sites infiltrated with fatty tissue. By age 40 years, the marrow
lineage potential from lymphoid to myeloid development. This may in sternum, ribs, pelvis and vertebrae is composed of equal amounts
contribute to an observed relative increase in neutrophils and decrease of hematopoietic tissue and fat and cellularity declines gradually there-
in lymphocytes in the blood of older people. 157 after. By age 65 years, marrow cellularity is estimated to be approxi-
There is an intrinsic change in HSCs with age, most notably result- mately 30 percent, 135,136 with a corresponding increase in marrow fat.
ing in a shift in lineage potential from lymphoid to myeloid development. Age-associated imbalanced bone remodeling and osteoporosis results
This may contribute to a relative increase in neutrophils and decrease in in decreased trabecular bone which itself may contribute to diminished
157
lymphocytes in the blood of older persons. As HSCs age, they accumu- hematopoiesis. The presence of fat correlates with the occurrence and
158
late genotypic (mutational) and phenotypic alterations. Indeed, human severity of osteoporosis, both of which are evident with aging. Several
159
stem-progenitor cells from healthy volunteers were found to accumu- age-related qualitative changes have been identified in hematopoietic
late 13 exonic (private) mutations per year of age. 157a Current opinion cells, including skewed X-chromosome inactivation, telomere short-
is that such changes are responsible for the development of immune ening, 160–162 accumulation of mitochondrial DNA mutations, 163,164 and
Bone marrow Thymus
Cellularity Relative thymic mass
100 100
% Marrow occupied by hematopoietic cells 60 % Peak thymic mass 60
80
80
40
40
20
20
0
0 20 40 60 80 100 0 0 20 40 60 80 100
Age (years) Age (years)
Clinical consequences*
Mild anemia (unexplained) Mild immune deficiency
?Increased GVHD as marrow donor Increased susceptibility to herpes zoster
Tuberculosis reactivation
?Predisposition to cancer
*Consequences of aging in the bone marrow and thymus in the absence of disease.
Figure 9–1. Aging of marrow and thymus. Marrow cellularity declines from birth in a manner comparably to thymic mass. This is reflected histolog-
ically by the increased presence of fat. The clinical consequences of these age-associated changes, in the absence of disease, are a mild anemia and
immune deficiency. The latter is reflected by an increased predisposition to certain infections (e.g., herpes zoster or reactivation of latent tuberculosis)
and possibly to the increased predisposition to cancer. GVHD, graft-versus-host disease.
Kaushansky_chapter 09_p0129-0142.indd 133 17/09/15 6:16 pm