Page 2299 - Williams Hematology ( PDFDrive )
P. 2299
2274 Part XII: Hemostasis and Thrombosis Chapter 133: Venous Thrombosis 2275
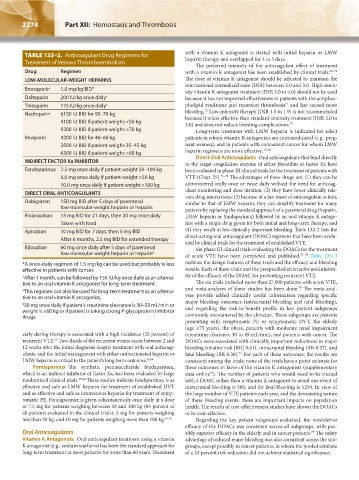
TABLE 133–2. Anticoagulant Drug Regimens for with a vitamin K antagonist is started with initial heparin or LMW
heparin therapy and overlapped for 4 to 5 days.
Treatment of Venous Thromboembolism
The preferred intensity of the anticoagulant effect of treatment
Drug Regimen with a vitamin K antagonist has been established by clinical trials. 69–72
LOW-MOLECULAR-WEIGHT HEPARINS The dose of vitamin K antagonist should be adjusted to maintain the
international normalized ratio (INR) between 2.0 and 3.0. High-inten-
Enoxaparin 1.0 mg/kg BID* sity vitamin K antagonist treatment (INR 3.0 to 4.0) should not be used
Dalteparin 200 IU/kg once daily † because it has not improved effectiveness in patients with the antiphos-
71
Tinzaparin 175 IU/kg once daily ‡ pholipid syndrome and recurrent thrombosis and has caused more
72
Nadroparin 6150 IU BID for 50–70 kg bleeding. Low-intensity therapy (INR 1.5 to 1.9) is not recommended
4100 IU BID if patient weighs <50 kg because it is less effective than standard-intensity treatment (INR 2.0 to
3.0) and does not reduce bleeding complications.
70
9200 IU BID if patient weighs >70 kg Long-term treatment with LMW heparin is indicated for select
Reviparin 4200 IU BID for 46–60 kg patients in whom vitamin K antagonists are contraindicated (e.g., preg-
3500 IU BID if patient weighs 35–45 kg nant women), and in patients with concurrent cancer for whom LMW
6300 IU BID if patient weighs >60 kg heparin regimens are more effective. 67,68
Direct Oral Anticoagulants Oral anticoagulants that bind directly
INDIRECT FACTOR Xa INHIBITOR to the target coagulation enzyme of either thrombin or factor Xa have
Fondaparinux 7.5 mg once daily if patient weight 50–100 kg been evaluated in phase III clinical trials for the treatment of patients with
5.0 mg once daily if patient weight <50 kg VTE (Chap. 25). 73–78 The advantages of these drugs are: (1) they can be
10.0 mg once daily if patient weight >100 kg administered orally once or twice daily without the need for anticoag-
DIRECT ORAL ANTICOAGULANTS ulant monitoring and dose titration; (2) they have fewer clinically rele-
vant drug interactions; (3) because of a fast onset of anticoagulant action,
Dabigatran 150 mg BID after 5 days of parenteral similar to that of LMW heparin, they can simplify treatment for many
low-molecular-weight heparin or heparin patients by replacing the standard approach of a parenteral drug (heparin,
Rivaroxaban 15 mg BID for 21 days, then 20 mg once daily LMW heparin or fondaparinux) followed by an oral vitamin K antago-
Taken with food nist with a single drug given for both initial and long-term therapy; and
Apixaban 10 mg BID for 7 days, then 5 mg BID (4) they result in less clinically important bleeding. Table 133-2 lists the
After 6 months, 2.5 mg BID for extended therapy direct-acting oral anticoagulant (DOAC) regimens that have been evalu-
ated by clinical trials for the treatment of established VTE.
Edoxaban 60 mg once daily after 5 days of parenteral Six phase III clinical trials evaluating the DOACs for the treatment
low-molecular-weight heparin or heparin §
of acute VTE have been completed and published. 73–78 Table 133–3
*A once-daily regimen of 1.5 mg/kg can be used but probably is less outlines the design features of these trials and the efficacy and bleeding
effective in patients with cancer. results. Each of these trials met the prespecified criteria for noninferior-
† After 1 month, can be followed by 150 IU/kg once daily as an alterna- ity of the efficacy of the DOAC for preventing recurrent VTE.
tive to an oral vitamin K antagonist for long-term treatment. The six trials included more than 27,000 patients with acute VTE,
79
‡ This regimen can also be used for long-term treatment as an alterna- and meta-analyses of these studies has been done. The meta-anal-
tive to an oral vitamin K antagonist. yses provide added clinically useful information regarding specific
§ 30 mg once daily if patient’s creatinine clearance is 30–50 mL/min or major bleeding outcomes (intracranial bleeding and fatal bleeding),
weight is ≤60 kg or if patient is taking strong P-glycoprotein inhibitor and regarding the risk-to-benefit profile in key patient subgroups
drugs. commonly encountered by the clinician. These subgroups are patients
presenting with symptomatic PE or symptomatic DVT, the elderly
(age ≥75 years), the obese, patients with moderate renal impairment
early during therapy is associated with a high incidence (25 percent) of (creatinine clearance 30 to 49 mL/min), and patients with cancer. The
recurrent VTE. Two-thirds of the recurrent events occur between 2 and DOACs were associated with clinically important reductions in major
63
12 weeks after the initial diagnosis despite treatment with oral anticoag- bleeding (relative risk [RR] 0.61), intracranial bleeding (RR 0.37), and
ulants, and the initial management with either unfractionated heparin or fatal bleeding (RR 0.36). For each of these outcomes, the results are
79
LMW heparin is critical to the patient’s long-term outcome. 63,64 consistent among the trials; none of the trials have a point estimate for
Fondaparinux The synthetic pentasaccharide fondaparinux, these outcomes in favor of the vitamin K antagonists (supplementary
which is an indirect inhibitor of factor Xa, has been evaluated by large data online ). The number of patients who would need to be treated
79
randomized clinical trials. 65,66 These studies indicate fondaparinux is as with a DOAC rather than a vitamin K antagonist to avoid one event of
effective and safe as LMW heparin for treatment of established DVT intracranial bleeding is 588, and for fatal bleeding is 1250. In view of
and as effective and safe as intravenous heparin for treatment of symp- the large number of VTE patients each year, and the devastating nature
tomatic PE. Fondaparinux is given subcutaneously once daily at a dose of these bleeding events, these are important impacts on population
of 7.5 mg for patients weighing between 50 and 100 kg (85 percent of health. The results of cost-effectiveness studies have shown the DOACs
all patients evaluated in the clinical trials), 5 mg for patients weighing to be cost-effective.
less than 50 kg, and 10 mg for patients weighing more than 100 kg. 65,66 Regarding the key patient subgroups evaluated, the noninferior
efficacy of the DOACs was consistent across all subgroups, with pos-
Oral Anticoagulants sibly superior efficacy in the elderly and in cancer patients. The safety
79
Vitamin K Antagonists Oral anticoagulant treatment using a vitamin advantage of reduced major bleeding was also consistent across the sub-
K antagonist (e.g., sodium warfarin) has been the standard approach for groups, except possibly in cancer patients, in whom the pooled estimate
long-term treatment in most patients for more than 60 years. Treatment of a 33 percent risk reduction did not achieve statistical significance.
Kaushansky_chapter 133_p2267-2280.indd 2274 9/18/15 10:53 AM