Page 2300 - Williams Hematology ( PDFDrive )
P. 2300
2274 Part XII: Hemostasis and Thrombosis Chapter 133: Venous Thrombosis 2275
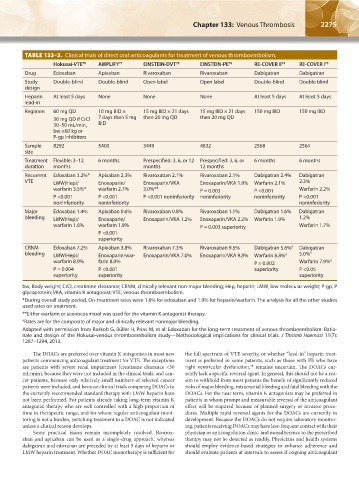
TABLE 133–3. Clinical trials of direct oral anticoagulants for treatment of venous thromboembolism.
Hokusai-VTE 78 AMPLIFY 77 EINSTEIN-DVT 75 EINSTEIN-PE 76 RE-COVER II 74 RE-COVER I 73
Drug Edoxaban Apixaban Rivaroxaban Rivaroxaban Dabigatran Dabigatran
Study Double-blind Double-blind Open label Open label Double-blind Double blind
design
Heparin At least 5 days None None None At least 5 days At least 5 days
lead-in
Regimen 60 mg QD 10 mg BID × 15 mg BID × 21 days 15 mg BID × 21 days 150 mg BID 150 mg BID
30 mg QD if CrCl 7 days then 5 mg then 20 mg QD then 20 mg QD
30–50 mL/min, BID
bw ≤60 kg or
P-gp inhibitors
Sample 8292 5400 3449 4832 2568 2564
size
Treatment Flexible: 3–12 6 months Prespecified: 3, 6, or 12 Prespecified: 3, 6, or 6 months 6 months
duration months months 12 months
Recurrent Edoxaban 3.2%* Apixaban 2.3% Rivaroxaban 2.1% Rivaroxaban 2.1% Dabigatran 2.4% Dabigatran
VTE LMW(Hep)/ Enoxaparin/ Enoxaparin/VKA Enoxaparin/VKA 1.8% Warfarin 2.1% 2.3%
warfarin 3.5%* warfarin 2.7% 3.0%** P = 0.003 P <0.001 Warfarin 2.2%
P <0.001 P <0.001 P <0.001 noninferiority noninferiority noninferiority P <0.001
noninferiority noninferiority noninferiority
Major Edoxaban 1.4% Apixaban 0.6% Rivaroxaban 0.8% Rivaroxaban 1.1% Dabigatran 1.6% Dabigatran
bleeding LMW(Hep)/ Enoxaparin/ Enoxaparin/VKA 1.2% Enoxaparin/VKA 2.2% Warfarin 1.9% 1.2%
warfarin 1.6% warfarin 1.8% P = 0.003 superiority Warfarin 1.7%
P <0.001
superiority
CRNM Edoxaban 7.2% Apixaban 3.8% Rivaroxaban 7.3% Rivaroxaban 9.5% Dabigatran 5.6% § Dabigatran
bleeding LMW(Hep)/ Enoxaparin/war- Enoxaparin/VKA 7.0% Enoxaparin/VKA 9.8% Warfarin 8.8% § 5.0% §
warfarin 8.9% farin 8.0% P = 0.002 Warfarin 7.9% §
P = 0.004 P <0.001 superiority P <0.05
superiority superiority superiority
bw, Body weight; CrCl, creatinine clearance; CRNM, clinically relevant non-major bleeding; Hep, heparin; LMW, low molecular weight; P-gp, P
glycoprotein; VKA, vitamin K antagonist; VTE, venous thromboembolism.
*During overall study period. On-treatment rates were 1.6% for edoxaban and 1.9% for heparin/warfarin. The analysis for all the other studies
used rates on treatment.
**Either warfarin or acenocoumarol was used for the vitamin K antagonist therapy.
§ Rates are for the composite of major and clinically relevant nonmajor bleeding.
Adapted with permission from Raskob G, Büller H, Prins M, et al: Edoxaban for the long-term treatment of venous thromboembolism: Ratio-
nale and design of the Hokusai-venous thromboembolism study—Methodological implications for clinical trials. J Thromb Haemost 11(7):
1287–1294, 2013.
The DOACs are preferred over vitamin K antagonists in most new the full spectrum of VTE severity, or whether “lead-in” heparin treat-
patients commencing anticoagulant treatment for VTE. The exceptions ment is preferred in some patients, such as those with PE who have
are patients with severe renal impairment (creatinine clearance <30 right ventricular dysfunction, remains uncertain. The DOACs cur-
78
mL/min), because they were not included in the clinical trials, and can- rently lack a specific reversal agent. In general, this should not be a rea-
cer patients, because only relatively small numbers of selected cancer son to withhold from most patients the benefit of significantly reduced
patients were included, and because clinical trials comparing DOACs to risks of major bleeding, intracranial bleeding and fatal bleeding with the
the currently recommended standard therapy with LMW heparin have DOACs. For the near term, vitamin K antagonists may be preferred in
not been performed. For patients already taking long-term vitamin K patients in whom prompt and measurable reversal of the anticoagulant
antagonist therapy who are well controlled with a high proportion of effect will be required because of planned surgery or invasive proce-
time in therapeutic range, and for whom regular anticoagulant moni- dures. Multiple rapid reversal agents for the DOACs are currently in
toring is not a burden, switching treatment to a DOAC is not indicated development. Because the DOACs do not require laboratory monitor-
unless a clinical reason develops. ing, patients receiving DOACs may have less-frequent contact with their
Some practical issues remain incompletely resolved. Rivarox- physician or anticoagulation clinic, and nonadherence to the prescribed
aban and apixaban can be used as a single-drug approach, whereas therapy may not be detected as readily. Physicians and health systems
dabigatran and edoxaban are preceded by at least 5 days of heparin or should employ evidence-based strategies to enhance adherence and
LMW heparin treatment. Whether DOAC monotherapy is sufficient for should evaluate patients at intervals to assess if ongoing anticoagulant
Kaushansky_chapter 133_p2267-2280.indd 2275 9/18/15 10:53 AM