Page 2331 - Williams Hematology ( PDFDrive )
P. 2331
2304 Part XII: Hemostasis and Thrombosis Chapter 135: Fibrinolysis and Thrombolysis 2305
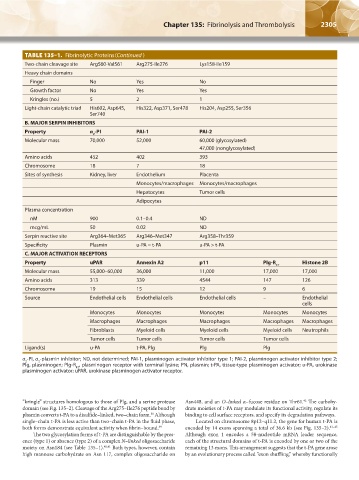
TABLE 135–1. Fibrinolytic Proteins (Continued )
Two-chain cleavage site Arg560-Val561 Arg275-Ile276 Lys158-Ile159
Heavy chain domains
Finger No Yes No
Growth factor No Yes Yes
Kringles (no.) 5 2 1
Light-chain catalytic triad His602, Asp645, His322, Asp371, Ser478 His204, Asp255, Ser356
Ser740
B. MAJOR SERPIN INHIBITORS
Property α -PI PAI-1 PAI-2
2
Molecular mass 70,000 52,000 60,000 (glycosylated)
47,000 (nonglycosylated)
Amino acids 452 402 393
Chromosome 18 7 18
Sites of synthesis Kidney, liver Endothelium Placenta
Monocytes/macrophages Monocytes/macrophages
Hepatocytes Tumor cells
Adipocytes
Plasma concentration
nM 900 0.1–0.4 ND
mcg/mL 50 0.02 ND
Serpin reactive site Arg364–Met365 Arg346–Met347 Arg358–Thr359
Specificity Plasmin u-PA = t-PA u-PA > t-PA
C. MAJOR ACTIVATION RECEPTORS
Property uPAR Annexin A2 p11 Plg-R Histone 2B
KT
Molecular mass 55,000–60,000 36,000 11,000 17,000 17,000
Amino acids 313 339 4544 147 126
Chromosome 19 15 12 9 6
Source Endothelial cells Endothelial cells Endothelial cells – Endothelial
cells
Monocytes Monocytes Monocytes Monocytes Monocytes
Macrophages Macrophages Macrophages Macrophages Macrophages
Fibroblasts Myeloid cells Myeloid cells Myeloid cells Neutrophils
Tumor cells Tumor cells Tumor cells Tumor cells
Ligand(s) u-PA t-PA, Plg Plg Plg
α -PI, α -plasmin inhibitor; ND, not determined; PAI-1, plasminogen activator inhibitor type 1; PAI-2, plasminogen activator inhibitor type 2;
2
2
Plg, plasminogen; Plg-R , plasminogen receptor with terminal lysine; PN, plasmin; t-PA, tissue-type plasminogen activator; u-PA, urokinase
KT
plasminogen activator; uPAR, urokinase plasminogen activator receptor.
“kringle” structures homologous to those of Plg, and a serine protease Asn448, and an O–linked α–fucose residue on Thr61. The carbohy-
42
domain (see Fig. 135–2). Cleavage of the Arg275–Ile276 peptide bond by drate moieties of t-PA may modulate its functional activity, regulate its
plasmin converts t-PA to a disulfide–linked, two–chain form. Although binding to cell surface receptors, and specify its degradation pathways.
38
single–chain t-PA is less active than two–chain t-PA in the fluid phase, Located on chromosome 8p12–q11.2, the gene for human t-PA is
both forms demonstrate equivalent activity when fibrin–bound. 39 encoded by 14 exons spanning a total of 36.6 kb (see Fig. 135–2). 43–45
The two glycosylation forms of t-PA are distinguishable by the pres- Although exon 1 encodes a 58-nucleotide mRNA leader sequence,
ence (type 1) or absence (type 2) of a complex N–linked oligosaccharide each of the structural domains of t-PA is encoded by one or two of the
moiety on Asn184 (see Table 135–1). 40,41 Both types, however, contain remaining 13 exons. This arrangement suggests that the t-PA gene arose
high mannose carbohydrate on Asn 117, complex oligosaccharide on by an evolutionary process called “exon shuffling,” whereby functionally
Kaushansky_chapter 135_p2303-2326.indd 2305 9/18/15 5:13 PM