Page 2333 - Williams Hematology ( PDFDrive )
P. 2333
2306 Part XII: Hemostasis and Thrombosis Chapter 135: Fibrinolysis and Thrombolysis 2307
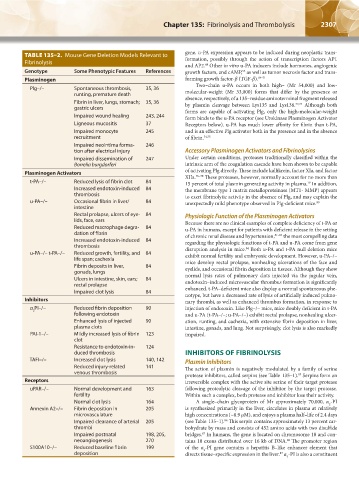
TABLE 135–2. Mouse Gene Deletion Models Relevant to gene. u-PA expression appears to be induced during neoplastic trans-
Fibrinolysis formation, possibly through the action of transcription factors AP1
68
and AP2. Other in vitro u-PA inducers include hormones, angiogenic
Genotype Some Phenotypic Features References growth factors, and cAMP, as well as tumor necrosis factor and trans-
55
Plasminogen forming growth factor-β (TGF-β). 69–71
Two–chain u-PA occurs in both high– (Mr 54,000) and low-
Plg–/– Spontaneous thrombosis, 35, 36
runting, premature death molecular-weight (Mr 33,000) forms that differ by the presence or
Fibrin in liver, lungs, stomach; 35, 36 absence, respectively, of a 135–residue aminoterminal fragment released
Although both
by plasmin cleavage between Lys135 and Lys136.
72,73
gastric ulcers forms are capable of activating Plg, only the high-molecular-weight
Impaired wound healing 243, 244 form binds to the u-PA receptor (see Urokinase Plasminogen Activator
Ligneous mucositis 37 Receptors below). u-PA has much lower affinity for fibrin than t-PA,
Impaired monocyte 245 and is an effective Plg activator both in the presence and in the absence
recruitment of fibrin. 74,75
Impaired neointima forma- 246
tion after electrical injury Accessory Plasminogen Activators and Fibrinolysins
Impaired dissemination of 247 Under certain conditions, proteases traditionally classified within the
Borrelia burgdorferi intrinsic arm of the coagulation cascade have been shown to be capable
Plasminogen Activators of activating Plg directly. These include kallikrein, factor XIa, and factor
These proteases, however, normally account for no more than
XIIa.
76–78
t-PA–/– Reduced lysis of fibrin clot 84 15 percent of total plasmin generating activity in plasma. In addition,
79
Increased endotoxin-induced 84 the membrane type 1 matrix metalloproteinase (MT1- MMP) appears
thrombosis to exert fibrinolytic activity in the absence of Plg, and may explain the
u-PA–/– Occasional fibrin in liver/ 84 unexpectedly mild phenotype observed in Plg-deficient mice. 80
intestine
Rectal prolapse, ulcers of eye- 84 Physiologic Function of the Plasminogen Activators
lids, face, ears Because there are no clinical examples of complete deficiency of t-PA or
Reduced macrophage degra- 84 u-PA in humans, except for patients with deficient release in the setting
dation of fibrin of chronic renal disease and hypertension, 81–83 the most compelling data
Increased endotoxin-induced 84 regarding the physiologic functions of t-PA and u-PA come from gene
thrombosis disruption analysis in mice. Both u-PA and t-PA null deletion mice
84
u-PA–/– t-PA–/– Reduced growth, fertility, and 84 exhibit normal fertility and embryonic development. However, u-PA–/–
life span; cachexia mice develop rectal prolapse, nonhealing ulcerations of the face and
Fibrin deposits in liver, 84 eyelids, and occasional fibrin deposition in tissues. Although they show
gonads, lungs normal lysis rates of pulmonary clots injected via the jugular vein,
Ulcers in intestine, skin, ears; 84 endotoxin–induced microvascular thrombus formation is significantly
rectal prolapse
Impaired clot lysis 84 enhanced. t-PA–deficient mice also display a normal spontaneous phe-
notype, but have a decreased rate of lysis of artificially induced pulmo-
Inhibitors nary thrombi, as well as enhanced thrombus formation, in response to
α PI–/– Reduced fibrin deposition 90 injection of endotoxin. Like Plg–/– mice, mice doubly deficient in t-PA
2
following endotoxin and u-PA (t-PA–/–; u-PA–/–) exhibit rectal prolapse, nonhealing ulcer-
Enhanced lysis of injected 90 ation, runting, and cachexia, with extensive fibrin deposition in liver,
plasma clots intestine, gonads, and lung. Not surprisingly, clot lysis is also markedly
PAI-1–/– Mildly increased lysis of fibrin 123 impaired.
clot
Resistance to endotoxin-in- 124
duced thrombosis INHIBITORS OF FIBRINOLYSIS
TAFI–/– Increased clot lysis 140, 142 Plasmin Inhibitors
Reduced injury-related 141 The action of plasmin is negatively modulated by a family of serine
venous thrombosis protease inhibitors, called serpins (see Table 135–1). Serpins form an
85
Receptors irreversible complex with the active site serine of their target protease
uPAR–/– Normal development and 163 following proteolytic cleavage of the inhibitor by the target protease.
fertility Within such a complex, both protease and inhibitor lose their activity.
Normal clot lysis 164 A single–chain glycoprotein of Mr approximately 70,000, α -PI
2
Annexin A2–/– Fibrin deposition in 205 is synthesized primarily in the liver, circulates in plasma at relatively
microvasculature high concentrations (~0.9 μM), and enjoys a plasma half–life of 2.4 days
86
Impaired clearance of arterial 205 (see Table 135–1). This serpin contains approximately 13 percent car-
thrombi bohydrate by mass and consists of 452 amino acids with two disulfide
Impaired postnatal 198, 205, bridges. In humans, the gene is located on chromosome 18 and con-
87
neoangiogenesis 270 tains 10 exons distributed over 16 kb of DNA. The promoter region
88
S100A10–/– Reduced baseline fibrin 199 of the α -PI gene contains a hepatitis B–like enhancer element that
2
deposition directs tissue–specific expression in the liver. α -PI is also a constituent
87
2
Kaushansky_chapter 135_p2303-2326.indd 2307 9/18/15 5:13 PM