Page 2418 - Williams Hematology ( PDFDrive )
P. 2418
2388 Part XIII: Transfusion Medicine Chapter 139: Preservation and Clinical Use of Platelets 2389
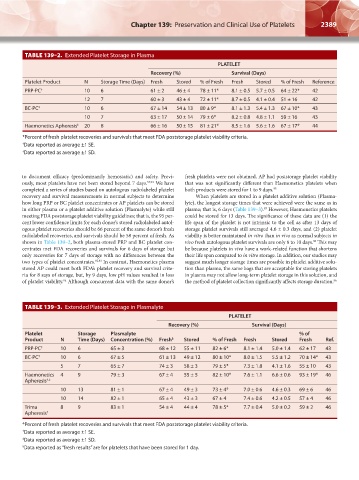
TABLE 139–2. Extended Platelet Storage in Plasma
PLATELET
Recovery (%) Survival (Days)
Platelet Product N Storage Time (Days) Fresh Stored % of Fresh Fresh Stored % of Fresh Reference
PRP-PC † 10 6 61 ± 2 46 ± 4 78 ± 11* 8.1 ± 0.5 5.7 ± 0.5 64 ± 22* 42
12 7 60 ± 3 43 ± 4 72 ± 11* 8.7 ± 0.5 4.1 ± 0.4 51 ± 16 42
BC-PC ‡ 10 6 67 ± 14 54 ± 13 80 ± 9* 8.1 ± 1.3 5.4 ± 1.3 67 ± 10* 43
10 7 63 ± 17 50 ± 14 79 ± 6* 8.2 ± 0.8 4.8 ± 1.1 59 ± 16 43
Haemonetics Apheresis ‡ 20 8 66 ± 16 50 ± 15 81 ± 21* 8.5 ± 1.6 5.6 ± 1.6 67 ± 17* 44
*Percent of fresh platelet recoveries and survivals that meet FDA poststorage platelet viability criteria.
† Data reported as average ±1 SE.
‡ Data reported as average ±1 SD.
to document efficacy (predominantly hemostatic) and safety. Previ- fresh platelets were not obtained, AP had poststorage platelet viability
ously, most platelets have not been stored beyond 7 days. 57,91 We have that was not significantly different than Haemonetics platelets when
completed a series of studies based on autologous radiolabeled platelet both products were stored for 1 to 9 days. 95
recovery and survival measurements in normal subjects to determine When platelets are stored in a platelet additive solution (Plasma-
how long PRP or BC platelet concentrates or AP platelets can be stored lyte), the longest storage times that were achieved were the same as in
in either plasma or a platelet additive solution (Plasmalyte) while still plasma; that is, 6 days (Table 139–3). However, Haemonetics platelets
93
meeting FDA poststorage platelet viability guidelines; that is, the 95 per- could be stored for 13 days. The significance of these data are (1) the
cent lower confidence limits for each donor’s stored radiolabeled autol- life span of the platelet is not intrinsic to the cell as after 13 days of
ogous platelet recoveries should be 66 percent of the same donor’s fresh storage platelet survivals still averaged 4.6 ± 0.3 days, and (2) platelet
radiolabeled recoveries, and survivals should be 58 percent of fresh. As viability is better maintained in vitro than in vivo as normal subjects in
96
shown in Table 139–2, both plasma-stored PRP and BC platelet con- vivo fresh autologous platelet survivals are only 8 to 10 days. This may
centrates met FDA recoveries and survivals for 6 days of storage but be because platelets in vivo have a work-related function that shortens
only recoveries for 7 days of storage with no differences between the their life span compared to in vitro storage. In addition, our studies may
two types of platelet concentrates. 92,93 In contrast, Haemonetics plasma suggest much longer storage times are possible in platelet additive solu-
stored AP could meet both FDA’s platelet recovery and survival crite- tion than plasma, the same bags that are acceptable for storing platelets
ria for 8 says of storage, but, by 9 days, low pH values resulted in loss in plasma may not allow long-term platelet storage in this solution, and
of platelet viability. Although concurrent data with the same donor’s the method of platelet collection significantly affects storage duration. 96
94
TABLE 139–3. Extended Platelet Storage in Plasmalyte
PLATELET
Recovery (%) Survival (Days)
Platelet Storage Plasmalyte % of
Product N Time (Days) Concentration (%) Fresh § Stored % of Fresh Fresh Stored Fresh Ref.
PRP-PC ‡ 10 6 65 ± 3 68 ± 12 55 ± 11 82 ± 6* 8.1 ± 1.4 5.0 ± 1.4 62 ± 17 43
BC-PC ‡ 10 6 67 ± 5 61 ± 13 49 ± 12 80 ± 10* 8.0 ± 1.5 5.5 ± 1.2 70 ± 14* 43
5 7 65 ± 7 74 ± 3 58 ± 3 79 ± 5* 7.3 ± 1.8 4.1 ± 1.6 55 ± 10 43
Haemonetics 4 9 79 ± 3 67 ± 4 55 ± 5 82 ± 10* 7.6 ± 1.1 6.6 ± 0.6 93 ± 19* 46
Apheresis †,‡
10 13 81 ± 1 67 ± 4 49 ± 3 73 ± 4* 7.0 ± 0.6 4.6 ± 0.3 69 ± 6 46
10 14 82 ± 1 65 ± 4 43 ± 3 67 ± 4 7.4 ± 0.6 4.2 ± 0.5 57 ± 4 46
Trima 8 9 83 ± 1 54 ± 4 44 ± 4 78 ± 5* 7.7 ± 0.4 5.0 ± 0.2 59 ± 2 46
Apheresis †
*Percent of fresh platelet recoveries and survivals that meet FDA poststorage platelet viability criteria.
† Data reported as average ±1 SE.
‡ Data reported as average ±1 SD.
§ Data reported as “fresh results” are for platelets that have been stored for 1 day.
Kaushansky_chapter 139_p2381-2392.indd 2389 9/18/15 2:23 PM